Contraceptive Implants
The contraceptive implant is the most effective reversible birth control method available. Implants, along with intrauterine devices (IUDs), are known as long-acting reversible contraception (LARCs) because they can be used to prevent pregnancy for several years and can be removed at any time. Implants are a relatively newer contraceptive method and have undergone substantial design modifications since their marred debut in the 1990s. The newest generation implant was introduced to the U.S. market in 2006, and remains the only contraceptive implant available in the U.S. Barriers to implant use include high up-front cost, lack of awareness and availability, and required insertion and removal by a trained health care provider. This fact sheet provides an overview of contraceptive implants including use, availability, and financing.
Background
In 1990, the Food and Drug Administration (FDA) approved Norplant, manufactured by Leiras Oy, the first subdermal contraceptive implant that was inserted under the skin of a woman’s upper arm by a health care provider. Made of silicone, it had six capsules containing levonorgestrel, a synthetic hormone, and was effective for up to five years. Following concerns about its effectiveness and lawsuits on behalf of women who experienced complications, Norplant’s distributor, Wyeth-Ayerst, discontinued its U.S. distribution in 2002.
In 2006, the FDA approved Implanon, a single, thin, plastic, etonogestrel-releasing rod manufactured by Organon USA (a division of Merck). The improved design and composition made Implanon easier and faster to insert and remove than first generation implants. In 2010, the manufacturer replaced Implanon with Nexplanon, which is designed to be radiopaque (visible through x-ray) and has an improved insertion device. It is FDA-approved for use up to three years, although some research indicates effectiveness beyond that period.1,2
With a 0.05% failure rate, the contraceptive implant is the most effective FDA-approved reversible contraceptive. Additionally, the implant removes the potential for user error and non-use associated with self-administered contraception because it is inserted by a provider and does not require any regular maintenance by the user.
Implants must be inserted and removed by a trained clinician who uses a special insertion device to place the implant just under the skin of the patient’s upper arm. The minor surgical procedure takes a few minutes and requires a local anesthetic and a small incision. After three years of use, the implant must be removed by a trained clinician. If the patient desires, a new implant can be placed at that time. Implants may be removed by a clinician at any time before three years and pregnancy can occur as soon as the first week following removal.
Contraceptive implants are safe for most women and can be inserted any time if it is reasonably certain she is not pregnant. Implants are primarily used for pregnancy prevention, but they can also be used to reduce menstrual cramps and make menstrual periods lighter. While there has been some concern about hormonal birth control for women who are breastfeeding, most findings show that progestin-only methods, such as the implant, do not appear to negatively affect breastfeeding outcomes.3 Some of the common side effects include irregular menstrual bleeding, headache, weight gain, acne, and breast pain, which may lead to discontinuation among some users.4 Less common risks associated with implant use include insertion and removal complications, ectopic pregnancy, and ovarian cysts. Although rare, some women who use the implant are at higher risk of developing blood clots, heart attack, or stroke.
Implant Use, Availability, and Outlook
Use
Because of their efficacy, continuation, and satisfaction rates, leading medical groups including the American College of Obstetricians and Gynecologists and the American Academy of Pediatrics have recommended the use of LARCs, like implants, for most women of reproductive age, including adolescents, and nulliparous and post-partum women. However, research demonstrates persistent misperceptions and a lack of awareness about implants. Although implant use in the U.S. has increased since it was first introduced in 1995, it is still lower than other contraceptive methods such as the IUD, pill, and sterilization.
In 2015-2017, the most recent years for which there are national data, about 4% of women ages 15-44 who currently use contraception used the implant, an increase from 1% in 2011-2013.5 At the same time, there has been a substantial rise in use of intrauterine devices (IUDs), which have also been promoted by several medical groups in recent years, from 10% to 14% during that same time period.
Implant users tend to be younger, lower-income, and have Medicaid coverage or are uninsured (compared to having private coverage) [Figure 1]. Possible explanations for the association of higher implant use among younger, less affluent women include the desire to avoid pregnancy for a longer period of time, lower maintenance and chance of user error, promotion of LARCs by medical organizations for adolescents, and availability at publicly-funded clinics.
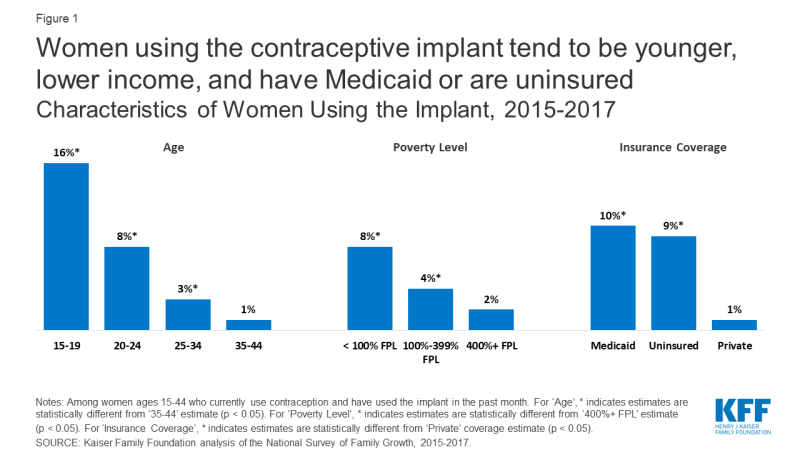
Figure 1: Women using the contraceptive implant tend to be younger, lower income, and have Medicaid or are uninsuredCharacteristics of Women Using the Implant, 2015-2017
Availability
Access to implants can depend, in part, on the clinician’s ability and willingness to offer them. The 2020 KFF National Physician Survey on Reproductive Health found that the majority (87%) of obstetricians and gynecologists (ob/gyns) offer the implant, though fewer (39%) offer same-day placement. Other research indicates that reasons for clinicians not offering the implant include lack of patient interest, insufficient training in this method, inadequate reimbursement, and provider beliefs.6,7,8
Furthermore, the FDA requires that clinicians complete specialized in-person training from the manufacturer. It should be noted that in addition to medical doctors, the training is open to advanced practice clinicians (such as nurses and physician assistants) who are authorized to perform implant insertions and removals in their practice jurisdiction. Although the 2-hour manufacturer training is offered at no cost to the provider, clinics may incur costs for travel and may encounter staffing challenges while their providers are being trained. It is unknown what percentage of providers have completed the manufacturer training, but one study found that approximately 90% of senior ob/gyn residents responding to a survey had received some implant training and felt comfortable inserting them.9 Additional clinical trainings for the implant are available from a variety of reproductive health organizations.
Federally Qualified Health Centers (FQHCs) are an important source of care for many low-income and uninsured women of reproductive age. However, provision of LARCs, including implants, has been challenging for some FQHCs due to high up-front costs of stocking them and limited training and staff capacity.10 A 2011 study found that only 36% of FQHCs offered contraceptive implants on-site. That share increased to 63% in 2017. Among these health centers, Title X grantees were more likely than non-Title X grantees to offer a broader range of contraceptive methods, including the implant (89% vs. 54%). A separate study found that specialized family planning clinics were more likely than primary care practices to offer long acting contraceptive methods. Furthermore, Planned Parenthood clinics were more likely than other types of publicly-funded clinics to offer a LARC method (98% vs. 69%-77%), and were more likely to provide same-day, on-site implant insertion, eliminating the need for women to make an additional visit or travel to another location. Research has found that reasons for clinics not offering same-day provision of implants include screening and waiting for STI test results (which is not required for implants), clinic flow, and scheduling issues.11
Outlook
There is currently no generic or therapeutically-equivalent version of Nexplanon available in the United States. Nexplanon’s manufacturer, Organon USA (a division of Merck), holds a patent on the device until 2027.
Although Merck does not indicate any current development of new or improved implants, the Contraceptive Pipeline Database, Calliope, lists several laboratory studies that are underway to develop a biodegradable contraceptive implant. None of these studies has moved beyond the pre-clinical development stage, but if successful, a biodegradable implant could reduce some of the barriers to implant use including the need to have it removed in a clinic and the procedure’s associated costs.
Insurance Coverage and Financing of Implants
The costs of implants have been a barrier to its use, for both patients and providers.12 Although implants are cost-effective and have been shown to become cost-saving within 3 years relative to short-acting methods, the up-front costs are high.13 The wholesale price for an implant is nearly $800, in addition to costs associated with insertion and removal.14 While many insurance plans have covered implants for years, prior to the passage of the Affordable Care Act (ACA), women with private insurance were likely to face out-of-pocket charges for the product as well as the associated visits. The ACA has eliminated these costs for many women, including those with private coverage. Medicaid has required coverage of family planning services without cost sharing in all states for decades, but the ACA expanded that to require coverage of all contraceptive methods for women enrolled in a Medicaid expansion program.15
Private Insurance
The ACA includes a requirement that most private insurance plans must cover at least one type of all 18 FDA-approved contraceptive methods for women as prescribed without cost sharing. This means that most private plans (small and large group, self-funded, and individually purchased plans) must cover the implant at no cost to policy holders. Research has found a 72% decline in average out-of-pocket spending on implants among women covered by one large insurer in the first year of the requirement.16 An analysis of claims data found that following the implementation of the ACA’s contraceptive coverage requirement in 2013, the median cost for implant insertions for women in private plans decreased to $0 and the percentage of women paying $0 for implants increased dramatically.17 Other analyses show increased utilization of LARCs among women with employer-sponsored insurance after implementation of this policy, suggesting that removing the high up-front cost of these methods may increase their use.18,19
Medicaid
Federal law requires Medicaid programs to cover family planning services and supplies without cost sharing, but there are variations in coverage or specific benefits between states and between different Medicaid populations. For women enrolled in traditional Medicaid programs that were in place prior to the passage of the ACA, coverage of implants is determined by each state program. In a 2015-2016 survey of 40 states and DC, all states reported covering all LARC methods (including IUDs and implants) in their traditional Medicaid programs. States may limit coverage to only certain brands or types or apply medical management protocols to restrict availability, which some states do. Additionally, in some states, LARC devices are tied to a specific patient, so if they are not used by the person for which they were ordered, the device must be discarded at a financial loss to the clinic (rather than being permitted for use by another patient). Recognizing the high (cost) effectiveness of LARCs, many states are pursuing policies to reduce barriers to provision, like reimbursing for insertion and removal, returning unused devices for credit, and providing hospitals with separate payments for post-partum LARC insertion.
Women who qualify for Medicaid under the ACA’s expansion of the program must receive coverage for the implant because the ACA requires these expansion programs to cover all FDA-approved methods for women without cost sharing, which is the same as the requirement for private insurance plans.
Currently, 25 states extend Medicaid coverage for family planning services, including contraception, to some uninsured women who do not qualify for full scope Medicaid. States retain the flexibility to decide whether implants are covered by these programs; however, the survey mentioned above found that all responding states that extend Medicaid for family planning also cover all LARCs.
Uninsured
The federal Title X Family Planning Program funds a network of clinics to provide family planning care to millions of low-income and uninsured women at reduced or no cost. In March 2019, the Trump administration finalized new regulations that made major changes to Title X, resulting in numerous grantees withdrawing from the program while lawsuits challenging the regulations make their way through the courts. The national program has emphasized increasing access to LARCs, especially to teens, by providing additional training for providers and clinics. Although on-site provision of the implant has increased in both Title X and non-Title X clinics in recent years, Title X clinics are more likely than non-Title X clinics to offer implants on-site. In 2017, 89% of Title X clinics offered implants on-site, an increase from 51% in 2011. While implant use among women visiting Title X clinics is still low, it increased from 1% in 2009 to 7% in 2018.
Recent initiatives around the country have found young women are very likely to choose the most effective methods of contraception when cost barriers are removed and contraceptive counseling is provided.20,21,22 The Contraceptive CHOICE Project offered women of reproductive age in the St. Louis, MO, region contraception of their choice with no cost sharing, leading to a reduction in teen births and repeat abortions among participants.23 Seventy-five percent of participants chose a LARC as their method of contraception, including 17% who chose the implant.24 While participants were more likely to choose the IUD than the implant, the percentage of participants choosing the implant is noteworthy in that it is higher than the percentage of women using the implant nationally. Research shows that satisfaction and continuation rates were relatively high among women who chose either method [Table 1]. High continuation rates among implant users have also been documented in populations with Medicaid coverage and coverage through an employer.25,26
| Table 1: Method Choice, Satisfaction, and Continuation Among Contraceptive CHOICE Project Participants | |||
| Implant | IUD | Other Methods | |
| Method choice1 | 17% | 58% | 25% |
| Satisfaction with method2 | 79% | 83% | 51% |
| Continuation after 12 months2 | 83% | 86% | 54% |
| 1 McNicholas, C, et al. (2014). The Contraceptive CHOICE Project round up: What we did and what we learned. Clinical Obstetrics and Gynecology 57(4). 2 Peipert, J, et al. (2011). Continuation and satisfaction of reversible contraception. Obstetrics & Gynecology 117(5). |
|||
***
Contraceptive implants are the most effective form of contraception, but use is relatively low, owing in part to: lack of awareness (they are newer to the market than many other methods); misperceptions about safety and efficacy; and high up-front costs. However, recent guidelines and initiatives have promoted implants and other long-acting reversible contraceptives as a first-line of defense against unintended pregnancy and an increasing share of health centers are offering them. Furthermore, the ACA’s requirement for coverage of contraceptive services and supplies without cost sharing removes cost barriers for millions of women, and policy changes in some state Medicaid programs are reducing the cost burden associated with stocking them in clinics. Programs that eliminate the high up-front financial barriers combined with contraceptive counseling may lead to increased use of highly-effective methods like the implant and contribute to a reduction in unintended pregnancies.
Endnotes
McNicholas, C, et al. (2015). Use of the etonogestrel implant and levonorgestrel intrauterine device beyond the U.S. Food and Drug Administration–approved duration. Obstetrics and Gynecology 125(3).
Ali, M, et al. (2016). Extended use up to 5 years of the etonogestrel-releasing subdermal contraceptive implant: comparison to levonorgestrel-releasing subdermal implant. Human Reproduction 31(11).
Phillips S, et al. (2016). Progestogen-only contraceptive use among breastfeeding women: a systematic review. Contraception 94(3).
Diedrich, J, et al. (2015). Three-year continuation of reversible contraception. American Journal of Obstetrics and Gynecology 213(5).
Kaiser Family Foundation analysis of the National Survey of Family Growth, 2011-2013 and 2015-2017.
Luchowski, A, et al. (2014). Obstetrician-Gynecologists and contraception: long-acting reversible contraception practices and education. Contraception 89(6).
Thompson, et al. (2018), Training contraceptive providers to offer intrauterine devices and implants in contraceptive care: a cluster randomized trial. American Journal of Obstetrics and Gynecology (218)6.
Morgan, et al. (2019). Health care provider attitudes about the safety of “quick start” initiation of long-acting reversible contraception for adolescents. Journal of Pediatric and Adolescent Gynecology (32)4.
Davis, S, et al. (2017). Familiarity with long-acting reversible contraceptives among obstetrics and gynecology, family medicine, and pediatrics residents: results of a 2015 national survey and implications for contraceptive provision for adolescents. Journal of Pediatric and Adolescent Gynecology 31(1).
Wood, S, et al. (2014). Accessibility of long-acting reversible contraceptives (LARCs) in Federally Qualified Health Centers (FQHCs). Contraception 89(2).
Biggs, M, Harper, C, & Brindis, C. (2015). California family planning health care providers' challenges to same-day long-acting reversible contraception provision. Obstetrics & Gynecology 126(2).
Morse, J, et al. (2012). Postabortion contraception: qualitative interviews on counseling and provision of long-acting reversible contraceptive methods. Perspectives on Sexual and Reproductive Health 44(2).
Trussell, J, et al. (2015). Achieving cost-neutrality with long-acting reversible contraceptive methods. Contraception 91(1).
Prescott G & Matthews C. (2013). Long‐acting reversible contraception: A review in special populations. Pharmacotherapy 34(1).
Grandfathered plans are exempt from this requirement. These are plans in existence prior to March 23, 2010, that have not made significant changes in coverage policies. In 2018, 16% of covered workers were in grandfathered plans.
Becker, N & Polsky, D. (2015). Women saw large decrease in out-of-pocket spending for contraceptives after ACA mandate removed cost sharing. Health Affairs 34(7).
Weisman, et al. (2019). ACA’s contraceptive coverage requirement: Measuring use and out-of-pocket spending. Health Affairs 38(9).
Carlin, C, Fertig, A, & Dowd, B. (2016). Affordable Care Act’s mandate eliminating contraceptive cost sharing influenced choices of women with employer coverage. Health Affairs 35(9).
Becker, N. (2018). The impact of insurance coverage on utilization of prescription contraceptives: Evidence from the Affordable Care Act. Journal of Policy Analysis and Management 37(3).
Sanders, J, et al. (2018). Contraceptive method use during the community-wide HER Salt Lake contraceptive initiative. American Journal of Public Health 108(4).
Ricketts, S, Klingler, G, & Schwalberg, R. (2014). Game change in Colorado: Widespread use of long-acting reversible contraceptives and rapid decline in births among young, low-income women. Perspectives on Sexual and Reproductive Health 46(3).
Secura, G, et al. (2010). The Contraceptive CHOICE Project: Reducing barriers to long-acting reversible contraception. American Journal of Obstetrics and Gynecology 203(2).
Peipert, J, Madden, T, Allsworth, J, & Secura, G. (2012). Preventing unintended pregnancies by providing no-cost contraception. Obstetrics and Gynecology 120(6).
McNicholas, C, et al. (2014). The Contraceptive CHOICE Project round up: What we did and what we learned. Clinical Obstetrics and Gynecology 57(4).
Romano, M, Toye, P, & Patchen, L. (2018). Continuation of long-acting reversible contraceptives among Medicaid patients. Contraception 98(2).
Berenson, A, et al. (2015). Complications and continuation rates associated with 2 types of long-acting contraception. American Journal of Obstetrics and Gynecology 212(6).
