Medicaid’s Most Costly Outpatient Drugs
Executive Summary
The launch of several expensive hepatitis C drugs over the past few years has ushered the topic of high cost prescription drugs back into the public’s and policymakers’ attention. With over 70 million beneficiaries, many of whom have complicated health needs, Medicaid is one of the largest providers of prescription drugs in the United States. State Medicaid agencies have a variety of mechanisms to help control outpatient drug spending that they have been using widely for the past decade. However, new prescription drugs are always coming to market, and the health needs of the Medicaid population change over time, especially as enrollment grows because of ACA Medicaid expansion. As a result, it is helpful for policy makers to understand which drugs used by the Medicaid program are most expensive. In this issue brief, we determine the 50 most costly drugs before rebates used by the Medicaid program from January 2014 through June 2015. We then examine reasons why these drugs are so costly, as well as exploring case studies on opioids, hepatitis C drugs, and the drug Abilify. Key findings include the following:
- Among the most commonly prescribed outpatient prescription drugs in Medicaid, the top five drugs are used for pain relief (hydrocodone-acetaminophen and ibuprofen), management of chronic illness (lisinopril and omeprazole), and antibiotics (amoxicillin). However, these drugs are not necessarily among the most costly used by Medicaid as many are inexpensive at the per prescription level.
- Several drugs that are very costly at the per prescription level reflect Medicaid’s role in caring for individuals with substantial health needs. These include drugs to treat hemophilia (NovoSeven RT, Koate-DVI, Feiba), multiple sclerosis (HP Acthar), and rare infant diseases (Adagen). However, most of these drugs are not commonly used among the Medicaid population.
- Aggregate drug costs to Medicaid reflect both frequency of use and per prescription cost. Among the most costly drugs in aggregate used by the Medicaid program are drugs used to treat costly illnesses for which Medicaid is a key source of coverage, including behavioral health conditions (Abilify and Vyvanse), hepatitis C (Sovaldi and Harvoni), and HIV (Truvada). Nearly three quarters of the 50 most costly drugs fall into five drug groups, the most prevalent of which is antivirals, which includes drugs used to treat HIV as well as hepatitis C drugs.
- 45 of the 50 most costly drugs fall into the high-cost category in part or primarily because they are frequently prescribed. Hydrocodone-acetaminophen and Suboxone, both opioids, a drug group which has garnered much public attention recently, fall into this category, as do several drugs used to treat ADHD.
- Many of the most costly drugs have some form of regulatory and consequently market exclusivity, thus enabling the manufacturers to charge a premium price for the drug at the prescription level. Twenty-two of the most costly drugs are particularly expensive at the prescription level, including the most costly drug before rebates used by Medicaid over this period, Abilify (an atypical antipsychotic).
As states continue to implement an array of measures to control Medicaid prescription drug costs, they are challenged to balance costs with access to drugs needed by beneficiaries. This challenge is not unique to Medicaid alone but is a common issue throughout the U.S. healthcare system, as reflected by the attention in general given to the high cost of prescription drugs. While this analysis may not reflect Medicaid’s net expenditures for a particular drug, as it is unable to account for drug rebates, it provides insights into which drugs are driving Medicaid prescription drug expenditures and why.
Issue Brief
Introduction
With its list price of $84,000 per treatment, the launch of the hepatitis C drug Sovaldi in December 2013 garnered the public’s and policymakers’ attention and brought into the spotlight the issue of high-cost prescription drugs in the U.S. Most Americans now believe that prescription drugs are too expensive.1 With over 70 million beneficiaries,2 the Medicaid program is larger than any other public or private insurer.3 Many Medicaid beneficiaries have poorer health than enrollees in private coverage4 and need prescription drugs to manage their medical conditions. As a result, Medicaid prescription drug spending is sizeable: in 2014, Medicaid spent $27.3 billion on outpatient drugs.5 Over the years, states have implemented an array of measures to control utilization and spending for prescription drugs.6
In this issue brief, we look at which outpatient prescription drugs were most expensive to Medicaid in 2014 and 2015 and explore why. As described in more detail in Appendix B, we merge state drug utilization data (available from the Centers for Medicare and Medicaid Services, or CMS) with Wolters Kluwer Clinical Drug Information (WKCDI) data to determine the 50 most costly outpatient drugs to the Medicaid program from January 2014 through June 2015 in terms of aggregate spending before rebates.7 Then, using WKCDI as well as data from the Food and Drug Administration (FDA), we focus on common attributes among them and discuss policy implications.
Background
The Medicaid outpatient prescription drug benefit is not a mandatory benefit, but all states provide this benefit in their Medicaid programs. Typically, a Medicaid beneficiary receives a prescription from their physician and fills it at a pharmacy. Medicaid either reimburses the pharmacy for the prescription or pays a capitation fee to a managed care company that reimburses the pharmacy for the prescription.8 States then collect rebates for the drugs prescribed to their beneficiaries from the manufacturers and share this with the federal government.9 As a result of the Medicaid Drug Rebate Program created by Congress in 1991, all drug manufacturers must enter into a rebate agreement with the Secretary of Health and Human Services in return for Medicaid reimbursement for their prescription drugs.10 The rebate program was put into place to control the rising cost of drugs within Medicaid.11
In the early 2000s, as Medicaid spending for prescription drugs grew, states implemented a number of cost control measures in this area, such as supplemental rebates, preferred drug lists, and generic substitution. With the implementation of Medicare Part D (and the shift of drug costs for dually eligible beneficiaries from Medicaid to Medicare), Medicaid saw a large decline in prescription drug spending, and state activity in this area slowed.12 However, after years of slow growth, Medicaid spending on outpatient prescription drugs jumped 24% in 2014 to $27.3 billion, or about 6 percent of Medicaid spending.13 This increase in the growth rate is in large part due to increased enrollment resulting from Medicaid expansion and new high cost hepatitis C drugs.14
In January 2016, CMS released the Covered Outpatient Drugs final rule, which details drug reimbursement provisions outlined in the ACA, some of which were put into place to help states control drug spending. One such provision is the requirement that states must base drug ingredient cost reimbursement to pharmacies on the actual price of the drug, as opposed to using list prices.15 The previously used ingredient cost reimbursements, referred to as “Estimated Acquisition Costs,” often relied on “commercially published reference prices” which did not necessarily represent the actual cost of the drug to the pharmacy and were therefore susceptible to being manipulated.16 Many states had already made the change to using actual acquisition costs prior to the final rule.17 Despite this policy change, some drug manufacturers may still have the leverage to charge a high price for a drug due to lack of competition.
As states continue to grapple with controlling Medicaid prescription drug spending, many are focusing on high-cost “specialty drugs.” In FY 2015, over two-thirds of the states reported actions to refine and enhance their pharmacy programs in response to new and emerging specialty and high-cost drug therapies.18 However, it is unclear what role specialty drugs—or other types of drugs— play in Medicaid prescription drug spending. This analysis examines which drugs are the most costly to Medicaid and looks at common attributes of the most costly drugs. This information may be helpful as states undertake policy actions to balance high costs with high needs.
What Makes a Drug a High Cost to the Medicaid Program?
Aggregate drug costs to Medicaid reflect both frequency of use and per prescription costs. Among the most commonly prescribed outpatient prescription drugs in Medicaid, the top five drugs are used for pain relief (hydrocodone-acetaminophen and ibuprofen), management of chronic illness (lisinopril and omeprazole), and antibiotics (amoxicillin) (see Appendix Table A3). However, these drugs are not necessarily among the most costly to Medicaid as many are inexpensive at the per prescription level. Similarly, many drugs that are quite costly at the per prescription level are not commonly used by Medicaid enrollees. These drugs, which include drugs to treat hemophilia (NovoSeven RT, Koate-DVI, Feiba), multiple sclerosis (HP Acthar), and rare infant diseases (Adagen), reflect Medicaid’s role in caring for individuals with substantial health needs.
When we examined which drugs were high cost by assessing total Medicaid spending by drug name for each outpatient drug provided from January 2014 through June 2015,19 Abilify, Sovaldi, Vyvanse, Harvoni, and Truvada top the list, respectively (see Appendix Table A1). These are drugs to treat costly illnesses for which Medicaid is a key source of coverage, including behavioral health conditions (Abilify and Vyvanse), hepatitis C (Sovaldi and Harvoni), and HIV (Truvada).
In Figure 1, we classify the 50 most costly (in aggregate) drugs in terms of how frequently prescribed and how expensive they are at the prescription level. If they are in the top 10th percentile of drugs by number of prescriptions, we identify them as “frequently prescribed.” If they were in the top 10th percentile of drugs by spending per prescription, we refer to them as an “expensive at the prescription level.”
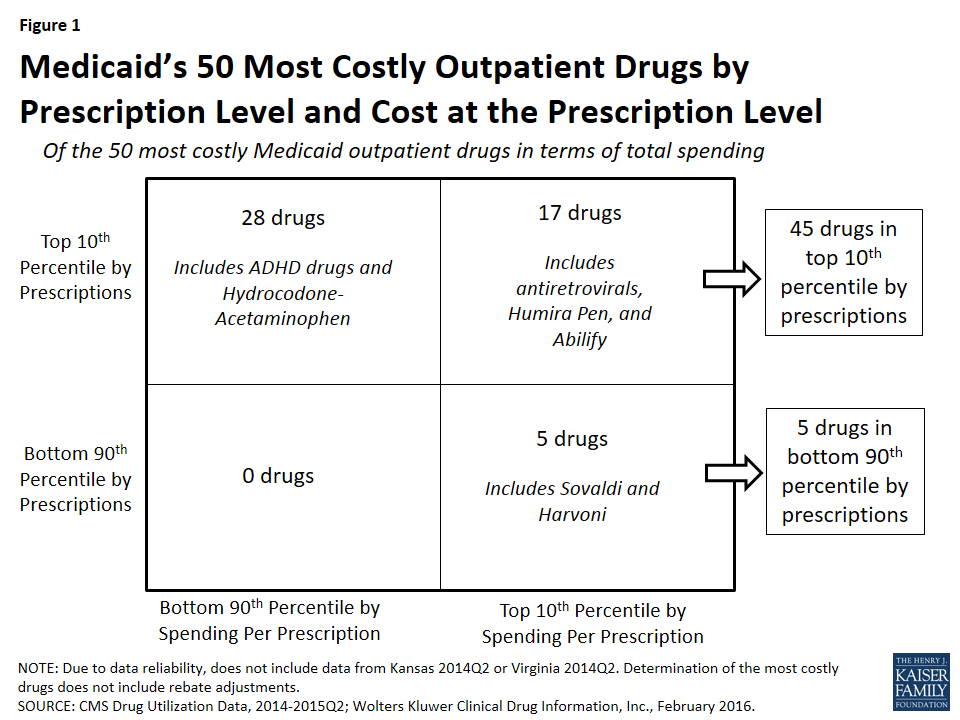
We found that of the 50 most costly drugs, 45 fall into the high-cost category at least in part because they are very frequently prescribed. Over half (28) are frequently prescribed but not expensive at the prescription level. Among others, these drugs include treatments for ADHD and hydrocodone-acetaminophen, the most frequently prescribed drug in Medicaid over this period (Appendix Table A3 and “Case Study A: Opioids”).
A smaller number of the 50 most costly drugs (17) are both frequently prescribed and expensive prescriptions. Seven antiretrovirals included among the 50 most costly drugs fall into this group (Truvada, Atripla, Prezista, Stribild, Complera, Reyataz, and Isentress). Among others, this category also includes Abilify, an atypical antipsychotic and the most costly drug to Medicaid (Appendix Table A1), and Humira Pen, a drug used to treat arthritis.
The five remaining drugs are expensive at the prescription level but not in the top tenth percentile by prescriptions, and thus we have not defined them as being frequently prescribed.20 Among others, these drugs include the hepatitis C agents Sovaldi and Harvoni.
Case Study A: Opioids
Two opioids are included in Medicaid’s 50 most costly drugs. Hydrocodone-acetaminophen (the generic version of Vicodin), an opioid, is the most frequently prescribed drug in Medicaid over the January 2014 through June 2015 period and is among the 50 most costly drugs over that period (Appendix Tables A1 and A3). Although it provides relief to those experiencing severe pain, it also may be addictive.Also included in the 50 most costly drugs is Suboxone, another opioid used for the treatment of opioid use disorder. Suboxone is the brand-name for the combination drug buprenorphine/naloxone and is intended to be taken as a pill under the tongue; if administered intravenously, the naloxone produces opioid withdrawal symptoms as a deterrent for misuse. Both hydrocodone-acetaminophen and Suboxone fall into the top 50 most costly drugs due to their wide use; neither is in the top 10th percentile of spending per prescriptions. In fact, hydrocodone-acetaminophen is the least expensive drug at the prescription level among the 50 most costly drugs.
As a drug class, opioids were the second most prescribed drug group over the period of study and the most prescribed drug group in 2014 (data not shown). This high level of opioid prescriptions reflects the high level of use of opioids in the U.S. overall, which has been drawing more and more concern in recent years. More than six in ten drug overdoses in the U.S. involve an opioid and since 1999, opioid deaths overall and prescription opioid deaths in particular have quadrupled.21 The high rate of use, including the misuse and abuse, of opioids affects a cross-section of society. More than half of Americans know someone who has died from opioids, has been addicted to them or knows someone who has been, or has taken painkillers that were not prescribed for them or knows someone who has.22
Medicaid patients are prescribed opioids at twice the rate as other patients, and their risk of overdose is three to six times higher than other patients.23 States and the federal government are taking action to address this public health crisis (see Policy Implications) and one of the ways they are doing so is through the expansion of medication-assisted treatments, which is the use of FDA-approved medications such as Suboxone24 in combination with behavioral therapy.
Which Drugs are High Cost to Medicaid?
As shown in Figure 2, 72% (36) of the 50 most costly drugs are in five drug groups. Antivirals are the most common drug group among the most costly drugs, accounting for 20% of the top 50 drugs. The antivirals comprise seven antiretrovirals (drugs that are used primarily in the treatment of HIV), two hepatitis C agents, and one other type of antiviral. Reflecting the Medicaid population’s serious health needs, antiretrovirals are widely used in the program.25 Antiretrovirals are also costly on a per prescription basis. Hepatitis C agents are also costly on a per prescription basis but are not as frequently prescribed (Figure 1).
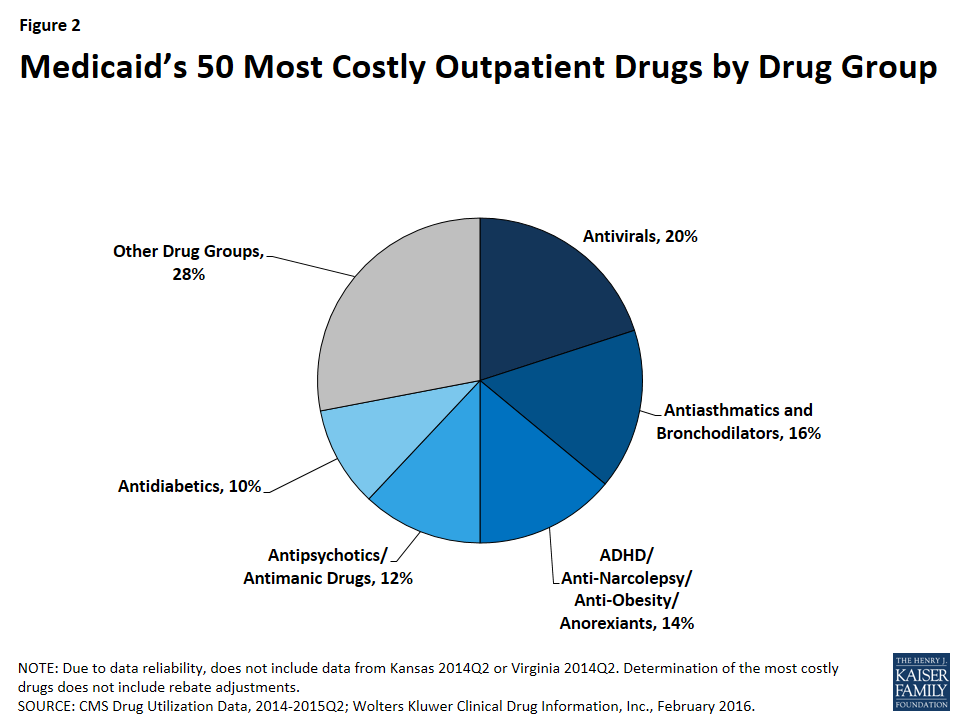
Antiasthmatics and bronchodilators are the second most common drug group in the 50 most costly drugs, accounting for 16% of the top 50 drugs. These are drugs used in the treatment of asthma and chronic obstructive pulmonary disease (COPD). None of these drugs are among the most expensive per prescription, but all are very frequently prescribed.26
With 14% (7), ADHD/Anti-Narcolepsy/Anti-Obesity/Anorexients are the third most common drug group in the most costly drugs. Although the drug group implies that these drugs can be used for a variety of conditions, the seven drugs within this drug group all have a common indication of attention deficit hyperactivity disorder (ADHD). Like the antiasthmatics and bronchodilators, all of these drugs are frequently prescribed and none of them are as expensive at the prescription level (Appendix Table A1). This high prescription rate is in part attributable to the high incidence rate of ADHD/ADD among children in general, among children in Medicaid, and among foster children in Medicaid.27
Twelve percent (6) of the most costly drugs are antipsychotics. Abilify, the most costly drug to Medicaid, is included within this group. Five out of the six antipsychotics in the 50 most costly drugs are both frequently prescribed and expensive at the prescription level (Appendix Table A1). Antipsychotics are FDA-approved to treat schizophrenia and bipolar disorder; however, they are also frequently prescribed “off-label” for other conditions.28
At ten percent, antidiabetics are the fifth most common drug group among the most costly drugs, with five drugs in this class falling into the top 50 most costly Medicaid drugs. Four of these five antidiabetics are insulins. None of the antidiabetics are in the top tenth percentile of drugs by spending per prescription, although the price of insulin has been increasing in recent years.29 All antidiabetics are very frequently prescribed, reflecting the high number of people in Medicaid who have diabetes: prior to Medicaid expansion, about four million Medicaid beneficiaries were diagnosed with diabetes.30
Case Study B: Hepatitis C Agents
Hepatitis C is a bloodborne virus that often remains asymptomatic in a person for many years or decades but ultimately can cause cirrhosis and cancer of the liver. As many as 5.2 million people in the U.S. have the hepatitis C.31 It disproportionately affects baby boomers and those enrolled in public coverage programs including Medicaid, Medicare, the VA, and state and federal prison systems.32 The prevalence of hepatitis C among Medicaid beneficiaries reflects that the Medicaid population in general has higher health needs than those who are privately insured.33 After the FDA approved it in December 2013,34 Gilead Sciences launched hepatitis C agent Sovaldi at a list price of $84,000 per treatment. Sovaldi is taken in combination with another antiviral, usually ribavirin.35 In October 2014, the FDA approved Gilead’s Harvoni, which does not require another drug to be taken in combination with it,36 and has a list price of $94,500 per treatment. Sovaldi and Harvoni are Direct-Acting Antivirals, which are “breakthrough-therapies,” as the previous standard treatment had a much lower cure rate as well as serious side-effects.37 Although they attracted attention in large part because of their high cost, Sovaldi and Harvoni are not the most expensive outpatient drugs to Medicaid at the prescription level on the market (Appendix Table A2).
In response to the high aggregate cost of these drugs, a majority of state Medicaid programs implemented various restrictions to accessing hepatitis C agents, many of which were based on the progress of the disease in the patient. States have also required alcohol and drug use screenings, consultation with a specialist, once-in-a-lifetime treatment maximums, and favorable viral response to the initial treatment.38 In 2014, only 2.4% of Medicaid enrollees with hepatitis C were able obtain to Sovaldi, in part due to states’ actions.39 State Medicaid programs are permitted to require prior authorization, implement prescription limits, and exclude drugs that are prescribed for a purpose other than their indication. However, they are not permitted to ultimately exclude access to a covered outpatient drug prescribed in accordance with its labeling for a treatment where that drug is an improvement compared to other covered outpatient drugs in terms of “safety, effectiveness, or clinical outcome[s].”40 CMS has expressed concern over the manner in which state Medicaid programs are restricting these drugs, reminding them that they may only restrict covered outpatient drugs in ways consistent with statute.41
A number of lawsuits have been filed against different state Medicaid agencies alleging that the restrictions on hepatitis C treatments are illegal and have caused harm to the plaintiffs.42 In May 2016, a federal court ordered the Washington state Medicaid program to lift the disease severity restrictions on hepatitis C treatments, marking the first time that a federal court declared such state Medicaid program restrictions to hepatitis C drugs illegal.43 Shortly after, in response to legal action and threatened legal action, Florida and then Delaware each announced in June of 2016 that Medicaid beneficiaries with hepatitis C would have access to needed medication, regardless of their stage of liver damage.44
How Does Market Exclusivity Affect Price?
In the absence of competition, a manufacturer may be able to price a drug higher. Patents and regulatory exclusivity, put into place as an incentive for innovation, are ways that a manufacturer can protect their product against competition. Patents have a twenty year duration, but manufacturers generally obtain them while their product is in preclinical and clinical trials, well before the FDA approves their product and well before the product launches. As a result, the duration remaining on the manufacturer’s original patent once the drug launches is usually much shorter than 20 years.45 Separate from the patent system, a manufacturer is also able to obtain regulatory exclusivity for their product from the FDA. Many of the 50 most costly drugs have some form of regulatory exclusivity.
Case Study C: Abilify
Abilify was Medicaid’s most costly drug from January 2014 through June 2015. It is an atypical antipsychotic,46 as are all of the antipsychotic drugs included in the 50 most costly drugs. The FDA approved Abilify in 2002.47 It is used in the treatment of schizophrenia, bipolar disorder, depression, and Tourette syndrome, and for symptoms of autistic disorder. Despite receiving two black-box warnings,48 ,49 doctors prescribed it and other atypical antipsychotics for additional diagnoses, such as anxiety and insomnia.50 Abilify was to lose patent protection in the spring of 2015, but shortly before that was to happen, the FDA approved it for the treatment of Tourette syndrome. Abilify’s manufacturer made efforts to stave off generic entry by trying to obtain an orphan drug designation for this new indication that would have ensured exclusivity through 2021, but ultimately they were unsuccessful, and the FDA-approved generic version of Abilify came onto the market in 2015.51 In the face of this loss of patent protection, its manufacturer increased the price of the drug, a strategy often seen before a brand drug’s patent expires.52
Brand Versus Generic Drugs
A brand drug is generally considered to be a drug that has received FDA approval after the manufacturer has proven the drug’s safety and efficacy. The FDA awards a regulatory exclusivity period of 3 or 5 years to brand drugs.53 Regulatory exclusivity provides the manufacturer with a degree of market exclusivity, enabling them to price the drug accordingly and providing incentive for them to market it as a non-commodity, which includes naming the drug with appealing brand name. Alternatively, a manufacturer can obtain FDA approval for their drug by proving that it is bioequivalent to a brand drug,54 skipping the long and expensive process of proving a drug is safe and effective. The FDA identifies these drugs as generic.55 They cannot enter the market while the corresponding brand still has exclusivity.56 Once generic drugs enter the market, the price of the drug usually falls due to competition.
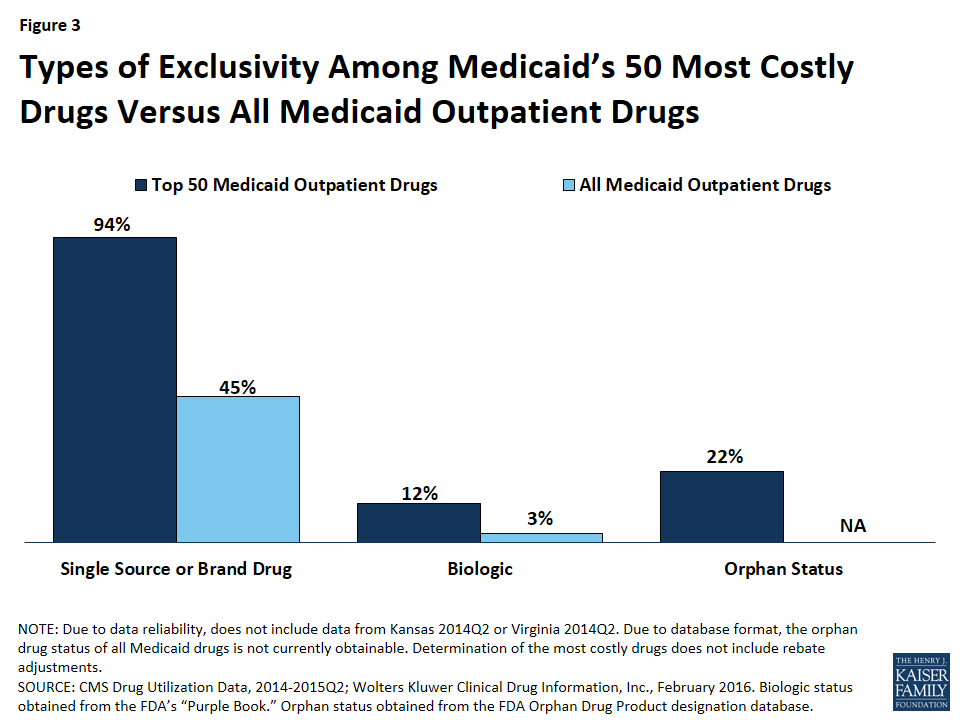
Compared to all drugs reimbursed through Medicaid from January 2014 through June 2015, we found a disproportionate number of the 50 most costly drugs are brands, as opposed to being generics (Figure 3).57 Ninety-four percent of the 50 most costly drugs were available as brand-name drugs, compared to 45% of all drugs reimbursed by Medicaid.
Biologics
A biologic is a drug that is derived from an animal or microorganism. It is more complex than traditional small-molecule drugs synthesized in a lab.58 Because biologics are structurally very different from small molecule drugs and are approved through a different process,59 there was not automatically a structure in place for generic approvals resulting in an absence of a generic market to commoditize biologic drugs. However, as part of the ACA,60 biologics now have 12 years of regulatory exclusivity,61 with an abbreviated pathway for the biosimilars, the biologic equivalent of a generic, now in place. Although biosimilars are expected to lower the price of the original biologic, they are not expected to lower it to that degree that generics lower the price of the original small-molecule brand drug.62 In March 2015, the FDA approved its first biosimilar, Zarxio, and the drug launched the following September.63
We found that 12% (6) of the 50 most costly drugs in Medicaid were biologics, compared with 3% of outpatient drugs reimbursed through Medicaid overall (Figure 3). The 6 biologics in the 50 most costly drugs are Humira Pen (used to treat psoriasis and some types of arthritis), Synagis (used to prevent serious lung disease), Advate (used in the treatment of hemophilia), Enbrel SureClick (used to treat psoriasis and some types of arthritis), Neulasta (used to treat possible side effects of chemotherapy), and NovoSeven RT (used in the treatment of hemophilia). All of these 6 biologics are in the top 5th percentile in terms of Medicaid spending per prescription. Twenty-nine of the 50 most expensive drugs by spending per prescription are biologics, showing that although these drugs are often very expensive, they do not necessarily appear as large budget items for Medicaid because of lower utilization (Appendix Table A2).
Orphan Drugs
The FDA provides orphan drug designations to drugs that treat fewer than 200,000 people in the U.S. or those that treat a disease for which the manufacturer does not expect to recover the cost of the drug.64 Having an orphan drug designation entitles the sponsor to many benefits,65 including a seven-year period of regulatory exclusivity associated with the drug’s indication. The 1982 Orphan Drug Act has generated an increase in the number of drug designations targeting rare diseases.66 However, some argue that it is being used to create blockbuster drugs, as manufacturers slice more common diseases into subtypes affecting fewer than 200,000 Americans and gain an orphan drug designation for a subtype, with the drug ultimately being used widely for other conditions.67
We found that 22% (11) of the 50 most costly drugs had achieved an orphan drug designation at some point (Figure 3). This includes Abilify, the most costly drug to Medicaid. Nine of these 11 drugs were among Medicaid’s top 10th percentile of most prescribed drugs.
Specialty Drugs
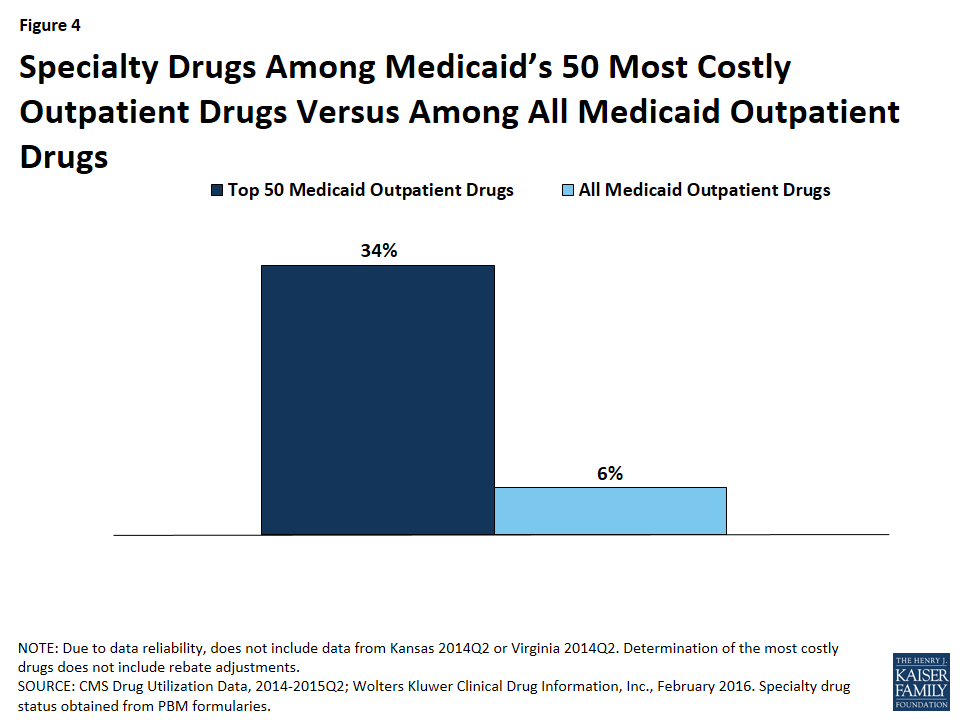
Although there is not one universally accepted definition, a specialty drug is generally considered to be a drug that requires difficult or unusual handling or is for a difficult-to-treat disease. Price is also often an indicator of a specialty drug.68 We found that a disproportionate number of drugs in the 50 most costly drugs are considered specialty drugs based on formulary review, with 34% (17) of the most costly drugs being considered specialty drugs, compared to 6% of all Medicaid covered outpatient drugs (Figure 3). Given that price factors into whether a drug is considered a specialty drug, it is not entirely surprising that so many of the most costly drugs are specialty drugs, and that many have a type of regulatory exclusivity. Of the 17 specialty drugs in the most costly drugs, none are multi-source generics (Appendix Table A1), and all but one are only available as branded single-source products with one labeler (data not shown). Six of the 17 specialty drugs are biologics, meaning that all biologics among the most costly drugs are considered specialty drugs (Appendix Table A1).
Policy Implications
In this analysis, we found that although all of the most costly drugs to Medicaid are frequently prescribed, expensive at the prescription level, or both; a majority are frequently prescribed. Access to prescription drugs is crucial for the treatment of many conditions found in the Medicaid population, which is more likely to have health issues than the privately insured. Although the prescription drug benefit is not mandatory for states, all state Medicaid programs provide it to their Medicaid beneficiaries. However, it can be expensive, and states are forced to grapple with the costs of the benefit. Additionally, although state Medicaid programs may know a new drug is coming to market, they may not have a sense for how expensive it will be until it reaches the market. States have taken a number of actions to control the budgetary effects of high cost drugs, including implementing new prior authorization requirements and negotiating higher supplemental rebates and lower prices for certain drugs.69
Balancing Cost with Access
Often, drugs that are high-priced are “orphan drugs” that treat rare diseases, which are allowed longer exclusivity so that the manufacturer can earn a profit on the drug. Sovaldi and Harvoni attracted attention because they were priced like orphan drugs,70 but they are for diseases that are prevalent in the Medicaid population. As these drugs were coming to market, nearly all states expressed concern about how the cost of this treatment would affect their Medicaid spending.71 However, while high cost, these drugs are cures for most patients; they are more effective than the previous standard drug treatment for the disease;72 and a full treatment of Sovaldi or Harvoni is less costly than a liver transplant, 73 for which hepatitis C is the leading cause.74 It is important to take a broad view when considering prescription drug costs, as many costly drugs prevent expensive emergency department visits and hospital stays. Regardless, states felt that it was not feasible to provide this drug to every beneficiary with hepatitis C immediately.75 In response, CMS published guidance reminding state Medicaid programs that certain utilization controls are permissible, but when doing so, states must ensure that they are in compliance with statute.76
In general, states can help control the overall costs of drugs by monitoring utilization and aiming to ensure that drugs are not overprescribed to patients. For example, states can require prior authorization or use a preferred drug list to control access. However, states generally must cover FDA-approved drugs for their medically accepted indications.77 States may not replace unreasonable limits on access to medically necessary drugs, such as those that are contrary to the professional standard of care. Many states have attempted to balance the public health need for hepatitis C drugs with their high cost through the implementation of prior authorization and other requirements.78 Spending and utilization for hepatitis C agents is lower than it would have been without these restrictions, but some beneficiaries who need these drugs are unable to access them, and a number of lawsuits have been filed against different state Medicaid agencies challenging state policies that limit access contrary to generally accepted standard of care among medical professionals.
Monitoring High Utilization
Many of the most costly drugs to Medicaid are so costly because they are frequently prescribed, including hydrocodone-acetaminophen, an opioid. While there are many medically necessary reasons to prescribe this drug, there is also a great deal of evidence to suggest overutilization of opioids. There is much that states can do to address the misuse of opioids, such as undertaking provider education; removing methadone79 from the preferred drug lists; establishing clinical criteria for obtaining a methadone prescription; requiring step therapy, prior authorization, or prescription quantity limits; using drug utilization review80 measures to identify potential misuse of opioids; increasing access to and use of prescription drug monitoring program data, and implementing patient review and restriction programs.81 States have acknowledged the severity of this public health crisis, and nearly all have prescription monitoring programs in place.82 There are hundreds of proposals in legislatures to regulate clinics and prescription behavior.83 The federal government has awarded money to health centers to focus on opioid abuse,84 and in March the Centers for Disease Control and Prevention released opioid prescription guidelines.85 Also as part of its collection of Medicaid quality measures, CMS is beginning to collect information on the use of opioids from multiple providers among non-cancer patients.86
Opioids are not the only drug at risk of being overprescribed. Three of the seven ADHD/Anti-Narcolepsy/Anti-Obesity/Anorexients are amphetamines, which are stimulants and have a black box warning of “high potential for abuse.”87 There is also general concern over the use of antipsychotics among children enrolled in Medicaid. As part of its collection of Medicaid and CHIP quality measures, CMS is beginning to collect information on children prescribed more than one antipsychotic at a time.88 As states monitor opioid and other drug groups as a way to address public health needs, they may realize budget savings due to lower utilization as well.
Promoting Innovation
Although policy makers are concerned with the cost of the prescription drug benefit, they also are concerned with beneficiaries having access to cures and treatments, and part of enabling this is incentivizing the pharmaceutical industry to research and bring to market new and needed drugs. In the U.S., innovation in the pharmaceutical industry is incentivized through regulatory exclusivity. This analysis has shown that many high cost drugs have some form of regulatory exclusivity. As Congress searches for ways to encourage innovation, they also remain aware of the varying ability for different payers to sustain these incentives. Nonetheless, although different payers face different parameters than Medicaid when paying for prescription drugs, this struggle to balance incentivizing innovation with actually being able to pay for the prescribed drugs is not unique to Medicaid alone, but a common challenge across the entire U.S. health care system.
Appendices: Appendix A: Tables
| Appendix Table A1: Medicaid’s 50 Most Costly Drugs, 2014Q1 – 2015Q2 | ||||||||
| Ranking | Drug Name | Specialty Drug | Biologic | Orphan Drug | Single-Source or Brand (B) vs. Multi-Source Generic (G) | Drug Group | Medicaid Spending Per Prescription Percentile* | Number of Prescriptions Percentile** |
| 1 | Abilify | X | B | Antipsychotics/Antimanic Agents | 90th | 99th | ||
| 2 | Sovaldi | X | B | Antivirals | 99th | 75th | ||
| 3 | Vyvanse | B | ADHD/Anti-Narcolepsy/Anti-Obesity/Anorexiants | 75th | 99th | |||
| 4 | Harvoni | X | B | Antivirals | 99th | 75th | ||
| 5 | Truvada | X | B | Antivirals | 90th | 95th | ||
| 6 | Lantus | B | Antidiabetics | 75th | 95th | |||
| 7 | Methylphenidate HCl ER | B & G | ADHD/Anti-Narcolepsy/Anti-Obesity/Anorexiants | 75th | 99th | |||
| 8 | Atripla | X | B | Antivirals | 95th | 95th | ||
| 9 | Advair Diskus | B | Antiasthmatic and Bronchodilator Agents | 75th | 95th | |||
| 10 | Lantus SoloStar | B | Antidiabetics | 75th | 95th | |||
| 11 | Seroquel XR | B | Antipsychotics/Antimanic Agents | 90th | 95th | |||
| 12 | Latuda | B | Antipsychotics/Antimanic Agents | 90th | 95th | |||
| 13 | Humira Pen | X | X | X | B | Anti-Inflammatory Analgesics | 95th | 90th |
| 14 | Adderall XR | B | ADHD/Anti-Narcolepsy/Anti-Obesity/Anorexiants | 75th | 95th | |||
| 15 | Suboxone | X | B | Opioid Analgesics | 75th | 95th | ||
| 16 | Invega Sustenna | B | Antipsychotics/Antimanic Agents | 90th | 95th | |||
| 17 | Flovent HFA | B | Antiasthmatic and Bronchodilator Agents | 75th | 95th | |||
| 18 | Lyrica | B | Anticonvulsants | 75th | 95th | |||
| 19 | Spiriva HandiHaler | X | B | Antiasthmatic and Bronchodilator Agents | 75th | 95th | ||
| 20 | ProAir HFA | B | Antiasthmatic and Bronchodilator Agents | 50th | 99th | |||
| 21 | Symbicort | B | Antiasthmatic and Bronchodilator Agents | 75th | 95th | |||
| 22 | Intuniv | B | ADHD/Anti-Narcolepsy/Anti-Obesity/Anorexiants | 75th | 95th | |||
| 23 | Synagis | X | X | B | Passive Immunizing Agents | 95th | 90th | |
| 24 | Prezista | X | B | Antivirals | 90th | 95th | ||
| 25 | Stribild | X | B | Antivirals | 95th | 90th | ||
| 26 | Advate | X | X | B | Hematological Agents | 99th | 75th | |
| 27 | Ventolin HFA | B | Antiasthmatic and Bronchodilator Agents | 50th | 99th | |||
| 28 | Focalin XR | B | ADHD/Anti-Narcolepsy/Anti-Obesity/Anorexiants | 75th | 95th | |||
| 29 | Strattera | X | B | ADHD/Anti-Narcolepsy/Anti-Obesity/Anorexiants | 75th | 95th | ||
| 30 | Qvar | B | Antiasthmatic and Bronchodilator Agents | 50th | 95th | |||
| 31 | Januvia | B | Antidiabetics | 75th | 95th | |||
| 32 | Humalog | B | Antidiabetics | 75th | 95th | |||
| 33 | Complera | X | B | Antivirals | 95th | 90th | ||
| 34 | Reyataz | X | B | Antivirals | 90th | 90th | ||
| 35 | Copaxone | X | X | B | Psychotherapeutic and Neurological Agents | 95th | 75th | |
| 36 | Duloxetine HCl | G | Antidepressants | 50th | 95th | |||
| 37 | Hydrocodone-Acetaminophen | B & G | Opioid Analgesics | 25th | 99th | |||
| 38 | Tamiflu | B | Antivirals | 75th | 95th | |||
| 39 | Nexium | B | Ulcer Drugs | 75th | 95th | |||
| 40 | Enbrel SureClick | X | X | X | B | Anti-Inflammatory Analgesics | 95th | 90th |
| 41 | Neulasta | X | X | X | B | Hematopoietic Agents | 95th | 90th |
| 42 | Novolog FlexPen | B | Antidiabetics | 75th | 95th | |||
| 43 | NovoSeven RT | X | X | X | B | Hematological Agents | 99th | 50th |
| 44 | Norditropin FlexPro | X | X | B | Endocrine and Metabolic Agents | 95th | 90th | |
| 45 | Isentress | X | B | Antivirals | 90th | 90th | ||
| 46 | Divalproex Sodium ER | G | Anticonvulsants | 50th | 95th | |||
| 47 | Seroquel | B | Antipsychotics/Antimanic Agents | 75th | 95th | |||
| 48 | Budesonide | X | B & G | Antiasthmatic and Bronchodilator Agents | 75th | 95th | ||
| 49 | Invega | B | Antipsychotics/Antimanic Agents | 90th | 90th | |||
| 50 | Amphetamine-Dextroamphet ER | G | ADHD/Anti-Narcolepsy/Anti-Obesity/Anorexiants | 50th | 95th | |||
| *We calculated average spending before rebates per prescription for each drug over the 2014Q1-2015Q2 period and identified notable percentiles. Medicaid Spending Per Prescription Percentile reflects how expensive each drug is before rebates at the prescription level, with respect to other Medicaid-reimbursed drugs, with 99th being the most expensive. **We summed the number of prescriptions for each drug over the 2014Q1-2015Q2 period and identified notable percentiles. Number of Prescriptions Percentile reflects how frequently prescribed each drug is, with respect to other Medicaid-reimbursed drugs, with 99th being the most prescribed. | ||||||||
| Appendix Table A2: Medicaid’s Most Expensive Drugs by Spending per Rx, 2014Q1 – 2015Q2 | |||||
| Ranking | Drug Name | Average Medicaid SpendingBefore Rebates Per Rx | Specialty Drug | Biologic | Drug Group |
| 1 | NovoSeven RT | $58,843 | X | X | Hematological Agents |
| 2 | Koate-DVI | $57,162 | X | X | Hematological Agents |
| 3 | Feiba | $48,366 | X | X | Hematological Agents |
| 4 | Adagen | $44,551 | Miscellaneous Biologicals | ||
| 5 | HP Acthar | $43,877 | X | Endocrine and Metabolic Agents | |
| 6 | Vimizim | $40,571 | X | X | Endocrine and Metabolic Agents |
| 7 | Myalept | $39,945 | X | X | Endocrine and Metabolic Agents |
| 8 | Chenodal | $39,556 | Gastrointestinal Agents | ||
| 9 | Carbaglu | $39,100 | X | Endocrine and Metabolic Agents | |
| 10 | Feiba NF | $36,418 | X | X | Hematological Agents |
| 11 | Ruconest | $33,659 | X | X | Hematological Agents |
| 12 | Gattex | $31,854 | X | Gastrointestinal Agents | |
| 13 | Cinryze | $31,640 | X | X | Hematological Agents |
| 14 | Firazyr | $31,103 | X | Hematological Agents | |
| 15 | Alprolix | $30,743 | X | X | Hematological Agents |
| 16 | Harvoni | $28,977 | X | Antivirals | |
| 17 | Novoeight | $28,897 | X | X | Hematological Agents |
| 18 | Alphanate/VWF Complex/Human | $28,571 | X | X | Hematological Agents |
| 19 | Juxtapid | $28,502 | Antihyperlipidemics | ||
| 20 | Rixubis | $28,330 | X | X | Hematological Agents |
| 21 | Cholbam | $27,422 | Gastrointestinal Agents | ||
| 22 | Berinert | $26,890 | X | X | Hematological Agents |
| 23 | Procysbi | $26,753 | X | Genitourinary Agents | |
| 24 | Sovaldi | $26,612 | X | Antivirals | |
| 25 | Orfadin | $26,562 | Endocrine and Metabolic Agents | ||
| 26 | Actimmune | $26,480 | X | X | Antineoplastics and Adjunctive Therapies |
| 27 | Cerdelga | $26,053 | X | Hematopoietic Agents | |
| 28 | Viekira Pak | $25,952 | X | Antivirals | |
| 29 | Ravicti | $25,840 | X | Endocrine and Metabolic Agents | |
| 30 | Hemofil M | $25,205 | X | X | Hematological Agents |
| 31 | Eloctate | $24,906 | X | X | Hematological Agents |
| 32 | Xyntha Solofuse | $23,846 | X | X | Hematological Agents |
| 33 | Kalydeco | $22,750 | X | Respiratory Agents | |
| 34 | Zavesca | $22,653 | X | Hematopoietic Agents | |
| 35 | Mononine | $22,647 | X | X | Hematological Agents |
| 36 | Ceprotin | $21,859 | X | X | Hematological Agents |
| 37 | Yervoy | $21,354 | X | X | Antineoplastics and Adjunctive Therapies |
| 38 | Olysio | $21,060 | X | Antivirals | |
| 39 | Kalbitor | $20,946 | X | X | Hematological Agents |
| 40 | Incivek | $20,575 | X | Antivirals | |
| 41 | BeneFIX | $20,019 | X | X | Hematological Agents |
| 42 | Kogenate FS | $20,017 | X | X | Hematological Agents |
| 43 | Kynamro | $19,731 | X | Antihyperlipidemics | |
| 44 | Corifact | $19,539 | X | X | Hematological Agents |
| 45 | Arcalyst | $19,014 | X | X | Anti-inflammatory Analgesics |
| 46 | Lumizyme | $18,855 | X | X | Endocrine and Metabolic Agents |
| 47 | Kogenate FS Bio-Set | $18,710 | X | X | Hematological Agents |
| 48 | AlphaNine SD | $18,389 | X | X | Hematological Agents |
| 49 | Ilaris | $18,133 | X | X | Anti-inflammatory Analgesics |
| 50 | Supprelin LA | $18,113 | X | Endocrine and Metabolic Agents | |
| NOTE: This table reflects Medicaid spending before rebates per prescription. A course of treatment for an illness may require multiple prescriptions. For example, a full treatment course for Hepatitis C agents Sovaldi and Harvoni often lasts three months and may require multiple prescriptions. | |||||
| Appendix Table A3: Medicaid’s Most Prescribed Drugs, 2014Q1 – 2015Q2 | ||
| Ranking | Drug Name | Drug Group |
| 1 | Hydrocodone-Acetaminophen | Opioid Analgesics |
| 2 | Amoxicillin | Penicillins |
| 3 | Ibuprofen | Anti-inflammatory Analgesics |
| 4 | Lisinopril | Antihypertensives |
| 5 | Omeprazole | Ulcer Drugs |
| 6 | Azithromycin | Macrolides |
| 7 | Gabapentin | Anticonvulsants |
| 8 | Fluticasone Propionate | Nasal Agents |
| 9 | Metformin HCl | Antidiabetics |
| 10 | Levothyroxine Sodium | Thyroid Agents |
| 11 | Cetirizine HCl | Antihistamines |
| 12 | Montelukast Sodium | Antiasthmatic and Bronchodilator Agents |
| 13 | ProAir HFA | Antiasthmatic and Bronchodilator Agents |
| 14 | Amlodipine Besylate | Calcium Channel Blockers |
| 15 | Albuterol Sulfate | Antiasthmatic and Bronchodilator Agents |
| 16 | Loratadine | Antihistamines |
| 17 | Ventolin HFA | Antiasthmatic and Bronchodilator Agents |
| 18 | Simvastatin | Antihyperlipidemics |
| 19 | Sertraline HCl | Antidepressants |
| 20 | Tramadol HCl | Opioid Analgesics |
| 21 | Oxycodone-Acetaminophen | Opioid Analgesics |
| 22 | Trazodone HCl | Antidepressants |
| 23 | Vitamin D (Ergocalciferol) | Vitamins |
| 24 | Alprazolam | Antianxiety Agents |
| 25 | Atorvastatin Calcium | Antihyperlipidemics |
| 26 | Hydrochlorothiazide | Diuretics |
| 27 | Clonazepam | Anticonvulsants |
| 28 | Citalopram Hydrobromide | Antidepressants |
| 29 | Cyclobenzaprine HCl | Musculoskelatal Therapy Agents |
| 30 | Ranitidine HCl | Ulcer Drugs |
| 31 | Fluoxetine HCl | Antidepressants |
| 32 | Amoxicillin-Potassium Clavulanate | Penicillins |
| 33 | Prednisone | Corticosteroids |
| 34 | Clonidine HCl | Antihypertensives |
| 35 | Risperidone | Antipsychotics/Antimanic Agents |
| 36 | Sulfamethoxazole-Trimethoprim | Anti-Infective Agents |
| 37 | Cephalexin | Cephalosporins |
| 38 | Metoprolol Tartrate | Beta Blockers |
| 39 | Methylphenidate HCl ER | ADHD/Anti-Narcolepsy/Anti-Obesity/Anorexiants |
| 40 | Quetiapine Fumarate | Antipsychotics/Antimanic Agents |
| 41 | Ondansetron HCl | Antiemetics |
| 42 | Triamcinolone Acetonide | Nasal Agents |
| 43 | Naproxen | Anti-inflammatory Analgesics |
| 44 | Vyvanse | ADHD/Anti-Narcolepsy/Anti-Obesity/Anorexiants |
| 45 | Furosemide | Diuretics |
| 46 | Oxycodone HCl | Opioid Analgesics |
| 47 | Zolpidem Tartrate | Hypnotics/Sedatives/Sleep Disorder Agents |
| 48 | Aspirin EC Low Dose | Nonnarcotic Analgesics |
| 49 | Lorazepam | Antianxiety Agents |
| 50 | Pantoprazole Sodium | Ulcer Drugs |
Appendices: Appendix B: Methodology
For our analysis of the 50 most costly drugs in the Medicaid program, we used 2014 and quarters one and two of 2015 State Drug Utilization Data available from CMS merged with data from Wolters Kluwer Clinical Drug Information, Inc (“WKCDI”).89 The State Drug Utilization Data is publicly available data used as part of the Medicaid Drug Rebate Program. This data provides information on the number of prescriptions, Medicaid spending, and cost-sharing for rebate-eligible Medicaid outpatient drugs at the National Drug Code (NDC) level. The WKCDI data provides product information for drug products. We accessed State Drug Utilization Data in February 2016 and used the most recent data available for all states. The WKCDI data is also from February 2016. The use of WKCDI data does not represent and should not be characterized as a WKCDI endorsement of any data, findings, or other content presented in this report.
We merged the State Drug Utilization Data and the WKCDI data at the NDC-level to consistently identify the drug name, as well as to incorporate brand versus generic status and the WKCDI Therapeutic Classifications System’s drug group. We classified single-source and multi-source, originator drugs as brand drugs. If a drug was available as both a brand and a generic, we categorized it as a brand when summarizing how many of the most costly drugs were brands and how many were generics. Using the Center for Drug Evaluation and Research List of Licensed Biological Products and the Center for Biologic Evaluation and Research List of Licensed Biological Products90 as of February 2016, we identified all biologics in the State Drug Utilization Data based on drug name. We looked up the orphan drug status of the 50 most costly drugs in Medicaid in the Orphan Drug Product designation database.91 To identify specialty drugs in the State Drug Utilization Data, we compiled a list using formularies from the top Medicaid pharmacy benefit managers.92 We identified specialty drugs using the drug name.
To determine the 50 most costly drugs to Medicaid, we summed total Medicaid spending before rebates by drug name over the 2014 quarter one through 2015 quarter two period. Due to data reliability, we were unable to include 2014 quarter two data from Kansas or Virginia. We also calculated average Medicaid spending per prescription and summed total prescriptions by drug name over this period. We ranked the drugs by spending per prescription and total prescriptions, calculated their percentiles, and identified a drug as “frequently prescribed” or “expensive at the prescription level” if it was in the top 10th percentile of either. We included the 50 most expensive drugs by spending per prescription and the 50 most prescribed drugs over the period in the Appendix tables. When reporting the former, we only included drugs with ten or more prescriptions to avoid any outliers in the data.
Limitations
This analysis does not include rebates, because this data is unavailable to the public at the NDC level. Rebates have a considerable effect on Medicaid drugs spending overall, but lower spending at the drug level at different rates.
Additionally, although Medicaid beneficiaries largely self-administer drugs that are prescribed in an outpatient setting, medical practitioners must administer some drugs. Although states are to collect drug rebates on all reimbursed outpatient drugs, regardless of whether they are physician- or self-administered, research has shown that not all states are collecting rebates on physician-administered drugs.93 Because biologics and other specialty drugs are often physician-administered, it is possible that the data reflects lower Medicaid spending and utilization of certain drugs of this kind.
Endnotes
- Bianca DiJulio, Jamie Firth, and Mollyann Brodie, Kaiser Health Tracking Poll: August 2015 (Washington DC: Kaiser Family Foundation, August 2015), https://modern.kff.org/health-costs/poll-finding/kaiser-health-tracking-poll-august-2015/. ↩︎
- This total also includes CHIP enrollment. See “Total Monthly Medicaid and CHIP Enrollment,” Kaiser Family Foundation, accessed July 6, 2016, https://modern.kff.org/health-reform/state-indicator/total-monthly-medicaid-and-chip-enrollment/. ↩︎
- U.S. Congress, Senate, Committee on Finance, The Price of Sovaldi and Its Impact on the U.S. Health Care System, 114th Congress, 1st session, 2015, 80, http://www.finance.senate.gov/imo/media/doc/1%20The%20Price%20of %20Sovaldi%20and%20Its%20Impact%20on%20the%20U.S.%20Health%20Care%20System%20(Full%20Report).pdf. ↩︎
- Julia Paradise and Rachel Garfield, What is Medicaid’s Impact on Access to Care, Health Outcomes, and Quality of Care? Setting the Record Straight on the Evidence (Washington DC: Kaiser Commission on Medicaid and the Uninsured, August 2013), https://modern.kff.org/medicaid/issue-brief/what-is-medicaids-impact-on-access-to-care-health-outcomes-and-quality-of-care-setting-the-record-straight-on-the-evidence/. ↩︎
- This includes rebates and spending through managed care. See “National Health Expenditure Accounts,” CMS, accessed July 6, 2016, https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NationalHealthAccountsHistorical.html. ↩︎
- Laura Snyder and Robin Rudowitz, Trends in State Medicaid Programs: Looking Back and Looking Ahead, (Washington DC: Kaiser Commission on Medicaid and the Uninsured, June 2016), https://modern.kff.org/medicaid/issue-brief/trends-in-state-medicaid-programs-looking-back-and-looking-ahead/. ↩︎
- In aggregate, the 50 most costly drugs compose about 40% of Medicaid drug spending before rebates over this period. ↩︎
- This is the case for beneficiaries not also dually enrolled in Medicare. Since 2006, Medicare Part D has paid for outpatient prescriptions of beneficiaries dually enrolled in Medicaid and Medicare. ↩︎
- 42 USC § 1396r-8(b)(1)(A). ↩︎
- 42 USC § 1396r-8(a)(1). ↩︎
- Brian Bruen and Katherine Young, What Drives Spending and Utilization on Medicaid Drug Benefits in States? (Washington DC: Kaiser Commission on Medicaid and the Uninsured, December 2014), http://files.kff.org/attachment/brief-what-drives-spending-and-utilization-on-medicaid-drug-benefits. ↩︎
- Vernon Smith, Kathleen Gifford, Eileen Ellis, Robin Rudowitz, Laura Snyder, and Elizabeth Hinton, Medicaid Reforms to Expand Coverage, Control Costs and Improve Care: Results from a 50-State Medicaid Budget Survey for State Fiscal Years 2015 and 2016, (Washington DC, Kaiser Commission on Medicaid and the Uninsured, October 2015), https://modern.kff.org/medicaid/report/medicaid-reforms-to-expand-coverage-control-costs-and-improve-care-results-from-a-50-state-medicaid-budget-survey-for-state-fiscal-years-2015-and-2016/. ↩︎
- This includes outpatient prescription drug spending for both managed care and fee-for-service, as well as incorporating rebates. Share of Medicaid spending does not include administrative or other non-personal care Medicaid spending. See National Health Expenditure Accounts, op. cit. ↩︎
- Medicaid and CHIP Payment and Access Commission, “June 2016 Report to Congress on Medicaid and CHIP,” (Washington DC, June 2016), https://www.macpac.gov/wp-content/uploads/2016/06/June-2016-Report-to-Congress-on-Medicaid-and-CHIP.pdf. ↩︎
- 42 CFR Part 447. ↩︎
- 77 Fed. Reg. 5318 (Feb. 2, 2012). ↩︎
- Brian Bruen and Katherine Young, Paying for Prescribed Drugs in Medicaid: Current Policy and Upcoming Changes, (Washington DC, Kaiser Commission on Medicaid and the Uninsured, May 2014), https://modern.kff.org/medicaid/issue-brief/paying-for-prescribed-drugs-in-medicaid-current-policy-and-upcoming-changes/ and Smith, Gifford, Ellis, Rudowitz, Snyder, and Hinton, op. cit. ↩︎
- Smith, Gifford, Ellis, Rudowitz, Snyder, and Hinton, op. cit. ↩︎
- Spending does not include rebates. For further details see the methodology section in the appendix. ↩︎
- Four out of five of these drugs are in the top quartile by prescriptions. The fifth is in the top 50th percentile by prescriptions. Although these drugs do not meet our definition of being “frequently prescribed,” they also are not rarely prescribed. ↩︎
- “Drug overdose deaths in the United States hit record numbers in 2014,” CDC, accessed July 6, 2016, http://www.cdc.gov/drugoverdose/epidemic/index.html. ↩︎
- Drew Altman, “Why Painkiller Addiction and Abuse are Rising Health-Care Priorities,” The Wall Street Journal, November 24, 2015, http://blogs.wsj.com/washwire/2015/11/24/why-painkiller-addiction-and-abuse-are-rising-health-care-priorities/. ↩︎
- Vikki Wachino, “Best Practices for Addressing Prescription Opioid Overdoses, Misuse and Addiction,” CMCS Informational Bulletin, CMS, January 28, 2016, https://www.medicaid.gov/federal-policy-guidance/downloads/cib-02-02-16.pdf. ↩︎
- The FDA has approved three medications for the treatment of opioid use disorder: buprenorphine, methadone, and naltrexone. Ibid. ↩︎
- Prior to Medicaid expansion, in FY 2011, over 200,000 Medicaid enrollees were diagnosed with HIV or AIDS. See “Medicaid Enrollment and Spending on HIV/AIDS”, Kaiser Family Foundation, accessed July 6, 2016, https://modern.kff.org/hivaids/state-indicator/enrollment-spending-on-hiv/. Medicaid plays an important role in the care of people with HIV or AIDS and Medicaid expansion has increased the number of HIV patients in care. See Jennifer Kates, Lindsey Dawson, Tresa Undem, and Kathleen Perry, Health Insurance Coverage for People with HIV Under the Affordable Care Act: Experiences in Five States, (Washington DC, Kaiser Family Foundation, December 2014), https://modern.kff.org/hivaids/issue-brief/health-insurance-coverage-for-people-with-hiv-under-the-affordable-care-act-experiences-in-five-states/. ↩︎
- Twenty-four percent of non-institutionalized Medicaid and CHIP enrollees had respiratory diseases in 2013 that incurred medical expenses. KFF analysis of 2013 Medical Expenditure Panel Survey (MEPS) household component, meps.ahrq.gov/mepsweb/. ↩︎
- Medicaid and CHIP Payment and Access Commission, “Behavioral Health in the Medicaid Program – People, Use, and Expenditures,” June 2015 Report to Congress on Medicaid and CHIP, (Washington DC, June 2015), https://www.macpac.gov/wp-content/uploads/2015/06/June-2015-Report-to-Congress-on-Medicaid-and-CHIP.pdf. ↩︎
- Louise Carton, Olivier Cottencin, Maryse Lapeyre-Mestre, Pierre Geoffroy, Jonathan Favre, Nicolas Simon, Regis Bordet, and Benjamin Rolland, “Off-Label Prescribing of Antipsychotics in Adults, Children, and Elderly Individuals: A Systematic Review of Recent Prescription Trends,” Current Pharmaceutical Design, 21, 23 (2015): 3280-3297, http://www.eurekaselect.com/132298/article. This practice of prescribing medications for uses not approved by the FDA is called “off-label prescribing” and is legal for non-controlled substances such as opioids. However, it is illegal for a pharmaceutical company to market the drug for off-label uses. See “Off-label Drug Use,” American Cancer Society, accessed July 6, 2016, http://www.cancer.org/treatment/treatmentsandsideeffects/treatmenttypes/chemotherapy/off-label-drug-use. ↩︎
- Ed Silverman, “Insulin prices have skyrocketed, putting drug makers on the defensive,” Stat News, April 5, 2016, https://www.statnews.com/pharmalot/2016/04/05/insulin-prices-skyrocketed-putting-drug-makers-defensive/. ↩︎
- KCMU and Urban Institute estimates based on data from FY 2011 MSIS. ↩︎
- Committee on Finance, op. cit., 1. ↩︎
- Ibid. ↩︎
- Paradise and Garfield, op. cit. ↩︎
- FDA, FDA Approves Sovaldi for Chronic Hepatitis C, December 6, 2013, http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm377888.htm ↩︎
- “What is Sovaldi?” Drugs.com, accessed July 6, 2016, http://www.drugs.com/sovaldi.html. ↩︎
- Gilead Sciences, U.S. Food and Drug Administration Approves Gilead’s Harvoni ® (Ledipasvir/Sofosbuvir), the First Once-Daily Single Tablet Regimen for the Treatment of Genotype 1 Chronic Hepatitis C, October 10, 2014, http://www.gilead.com/news/press-releases/2014/10/us-food-and-drug-administration-approves-gileads-harvoni-ledipasvirsofosbuvir-the-first-oncedaily-single-tablet-regimen-for-the-treatment-of-genotype-1-chronic-hepatitis-c ↩︎
- B.E. and A.R. v. Teeter, No. C16-227-JCC (W.D. Wa. May 27, 2016), https://today.law.harvard.edu/wp-content/uploads/2016/06/40-5-27-16-Order-Granting-Preliminary-Injunction.pdf. ↩︎
- Committee on Finance, op. cit., 87-88. ↩︎
- Ibid, 82. ↩︎
- 42 USC § 1396r-8. ↩︎
- “Assuring Medicaid Beneficiaries Access to Hepatitis C (HCV) Drugs,” Medicaid Drug Rebate Program Notice, CMS, November 5, 2015, https://www.medicaid.gov/Medicaid-CHIP-Program-Information/By-Topics/Benefits/Prescription-Drugs/Downloads/Rx-Releases/State-Releases/state-rel-172.pdf. ↩︎
- Micael Ollove, “Are States Obligated to Provide Expensive Hepatitis C Drugs?” Kaiser Health News, February 10, 2016, http://kffhealthnews.org/news/are-states-obligated-to-provide-expensive-hepatitis-c-drugs/. ↩︎
- Ed Silverman, “Washington State Told to Lift Restrictions on Hepatitis C Medicines,” Stat News, May 27, 2016, https://www.statnews.com/pharmalot/2016/05/27/washington-state-hepatitis-drug-prices/. ↩︎
- Associated Press, “Florida Changes Hep C Drug Policy For Medicaid After Lawsuit,” June 1, 2016, Tampa Bay Times, http://health.wusf.usf.edu/post/florida-changes-hep-c-drug-policy-medicaid-after-lawsuit#stream/0. Don Sapatkin, “Delaware will treat all Medicaid patients with hepatitis C,” June 9, 2016, http://articles.philly.com/2016-06-09/news/73647247_1_medicaid-patients-hepatitis-c-state-medicaid-program. ↩︎
- Robert Field, “Regulation of Drugs and Health Care Products” in Health Care Regulation in America: Complexity, Confrontation, and Compromise, (Oxford, England: Oxford University Press, 2007), 115-140. ↩︎
- Atypical antipsychotics are the second generation of antipsychotic drugs. They came onto the market in 1993, had fewer neurological side-effects than first generation antipsychotics, and were thought to be more effective in some schizophrenia symptoms. See Richard A. Friedman, “A Call for Caution on Antipsychotic Drugs,” New York Times, September 24, 2012, http://www.nytimes.com/2012/09/25/health/a-call-for-caution-in-the-use-of-antipsychotic-drugs.html. ↩︎
- “Orange Book: Approved Products with Therapeutic Equivalence Evaluations,” FDA, accessed July 6, 2016, http://www.accessdata.fda.gov/scripts/cder/ob/docs/querytn.cfm. ↩︎
- A boxed warning, commonly known as a “black box warning,” is a FDA warning added to the drug label describing serious or life-threatening risks. See “A Guide to Drug Safety Terms at the FDA,” FDA, accessed July 6, 2016, http://www.fda.gov/downloads/ForConsumers/ConsumerUpdates/UCM107976.pdf. It warns prescribing physicians to seriously consider the benefits of the drug given the known serious risks. Black box warnings can have an effect on the sales of the drug. See Lara Maggs and Aaron Kesselheim, “The Role of Black Box Warnings in Safe Prescribing Practices,” Health Affairs Blog, August 20 2014, http://healthaffairs.org/blog/2014/08/20/the-role-of-black-box-warnings-in-safe-prescribing-practices/. ↩︎
- Gardiner Harris, “Popular Drugs for Dementia Tied to Deaths,” New York Times, April 12, 2005, http://www.nytimes.com/2005/04/12/health/popular-drugs-for-dementia-tied-to-deaths.html?login=email. ↩︎
- Friedman, op. cit. ↩︎
- The FDA approved Abilify for treating children with Tourette syndrome in late 2014 and provided it with an orphan drug designation. Then, despite the sponsor not having applied for it, the FDA broadened Abilify’s designation to also include adults with Tourette syndrome, meaning that the population with the disease was too large for an orphan drug designation. Although Abilify’s manufacturer contested this, saying that the FDA could not provide a drug with a designation for which the sponsor had not applied, they were not successful in their bid. See Ed Silverman, “FDA Approves Generic Abilify After Unusual Legal Battle with Otsuka,” The Wall Street Journal, April 29, 2015, http://blogs.wsj.com/pharmalot/2015/04/29/fda-approves-generic-abilify-after-unusual-legal-battle-with-otsuka-2/. ↩︎
- The 2014 Drug Trend Report, (Express Scripts, March 2015). ↩︎
- If the New Drug Application (NDA) is for a drug with an active ingredient that the FDA has previously approved, but this drug is a new dosage form, new indication, or is now available as over-the-counter, the FDA approval grants a 3-year regulatory exclusivity period. If the drug contains chemical entities that have not previously been submitted to the FDA, the FDA generally grants a 5-year regulatory exclusivity period. The FDA provides an additional six months of exclusivity to sponsors who run pediatric studies on the submitted drug. See John Thomas, The Role of Patents and Regulatory Exclusivities in Pharmaceutical Innovation, (Washington DC: Congressional Research Service, January 7, 2013), http://www.ipmall.info/hosted_resources/crs/R42890_130107.pdf. ↩︎
- To obtain FDA approval for a generic drug, a manufacturer must show that the drug is identical to the brand in “dosage form, safety, strength, route of administration, quality, performance characteristics and intended use.” “What Are Generic Drugs?” FDA, accessed July 6, 2016, http://www.fda.gov/drugs/resourcesforyou/consumers/buyingusingmedicinesafely/understandinggenericdrugs/ucm144456.htm. ↩︎
- Ibid. ↩︎
- The Drug Price Competition and Patent Term Restoration Act of 1984, usually referred to as the Hatch-Waxman Act, created this framework of bringing generic drugs to market by allowing sponsors to file Abbreviated New Drug Applications (ANDAs) where they proved bioequivalence. At the same time, the Hatch-Waxman Act incentivized innovation by rewarding sponsors of new molecular entities and other brand drugs with varying lengths of regulatory exclusivity. ↩︎
- We considered a drug a brand-name drug if it was single-source, or if it was the originator product, but is now available from multiple sources. ↩︎
- “Frequently Asked Questions About Therapeutic Biological Products,” FDA, accessed July 6, 2016, http://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ ApprovalApplications/TherapeuticBiologicApplications/ucm113522.htm. ↩︎
- A manufacturer obtains FDA approval for a biologic through a Biologic License Approval (BLA) as opposed to an NDA or an ANDA. ↩︎
- Specifically, the part of the ACA that creates a framework for the approval of biosimilars and regulatory exclusivity to the original biologic is referred to as the Biologics Price Competition and Innovation Act. Biosimilars are not bioequivalent to the previously approved FDA biologic, in contrast to generic drugs which are bioequivalent to previously approved small-molecule drugs. Instead, biosimilars must be highly similar and interchangeable with the previously approved biologics. ↩︎
- It is possible that this may change in the future if the Trans-Pacific Partnership passes, as one point of negotiations between countries was the period of regulatory exclusivity for biologics. ↩︎
- Andrew Mulcahy, Zachary Predmore, and Soeren Mattke, “The Cost Savings Potential of Biosimilar Drugs in the United States,” (Rand Corporation, 2014), https://www.rand.org/content/dam/rand/pubs/perspectives/PE100/PE127/RAND_PE127.pdf. ↩︎
- Zarxio is a biosimilar to Neupogen, a biologic used to stimulate white blood cell growth in the treatment of cancer, bone marrow transplants, and chemotherapy. (“Neupogen,” Drugs.com, accessed July 6, 2016, http://www.drugs.com/neupogen.html.) However, Neupogen was not one of the 50 most costly drugs, nor was it one of the 50 most expensive drugs per prescription (Appendix Table A2). ↩︎
- Congress passed the Orphan Drug Act in 1982 to provide incentive for manufacturers to produce drugs for diseases rare enough that there had not previously been financial incentive to do so. Congress amended the law three times in the 80s, eventually arriving at these parameters for an orphan drug designation. See Gary Pulsinelli, “The Orphan Drug Act: What’s Right with It,” Santa Clara High Technology Law Journal, 5, 2. (January 1999): http://digitalcommons.law.scu.edu/cgi/viewcontent.cgi?article=1247&context=chtlj and “Developing Products for Rare Diseases & Conditions,” FDA, accessed July 6, 2016, http://www.fda.gov/ForIndustry/DevelopingProductsforRareDiseasesConditions/ucm2005525.htm. ↩︎
- These benefits also include federal funding for clinical trials and tax breaks, as well as other advantages. See Aaron Kesselheim, “Innovation and the Orphan Drug Act, 1983-2009: Regulatory and Clinical Characteristics of Approved Orphan Drugs” in Rare Diseases and Orphan Products: Accelerating Research and Development, (Washington DC: Institute of Medicine, 2010), 291-308, http://www.ncbi.nlm.nih.gov/books/NBK56187/. ↩︎
- Since 1983, the FDA has provided orphan drug designations to over 400 drugs that have gone to market. Between 1973 and 1983, there were fewer than 10. See “Developing Products for Rare Diseases & Conditions,” FDA, accessed July 6, 2016, http://www.fda.gov/ForIndustry/DevelopingProductsforRareDiseasesConditions/ucm2005525.htm. ↩︎
- Marty Makary, “One Pharma Fix: Limit the ‘Orphan Drug’ Incentives,” Wall Street Journal, Dec. 20, 2015, http://www.wsj.com/articles/one-pharma-fix-limit-the-orphan-drug-incentives-1450645511. ↩︎
- Bruen and Young, December 2014, op. cit. ↩︎
- Smith, Gifford, Ellis, Rudowitz, Snyder, and Hinton, op. cit. ↩︎
- Steve Miller, “Harvoni: Orphan-Drug Pricing for a Non-Orphan Drug,” (Express Scripts, October 2014), http://lab.express-scripts.com/lab/insights/specialty-medications/harvoni-orphan-drug-pricing-for-a-nonorphan-drug. ↩︎
- Vernon K. Smith, Kathleen Gifford, Eileen Ellis, Robin Rudowitz, and Laura Snyder, Medicaid in an Era of Health & Delivery System Reform: Results from a 50-State Medicaid Budget Survey for State Fiscal Years 2014 and 2015, (Washington DC, Kaiser Commission on Medicaid and the Uninsured, October 2014), https://modern.kff.org/medicaid/report/medicaid-in-an-era-of-health-delivery-system-reform-results-from-a-50-state-medicaid-budget-survey-for-state-fiscal-years-2014-and-2015/. ↩︎
- B.E. and A.R. v. Teeter, op. cit. ↩︎
- “Getting a Liver Transplant,” National Kidney Foundation, accessed July 6, 2016, https://www.kidney.org/atoz/content/livertx. ↩︎
- Committee on Finance, op. cit., 1. ↩︎
- Matt Salo, executive director of the National Association of State Medicaid Directors, conveyed the dilemma saying “We would be spending more on this one drug than all other drugs combined […] There isn’t the capacity to do that.” See Michael Ollove, “Are States Obligated to Provide Expensive Hepatitis C Drugs?” (Pew Trusts, February 2016), http://www.pewtrusts.org/en/research-and-analysis/blogs/stateline/2016/02/09/are-states-obligated-to-provide-expensive-hepatitis-c-drugs. ↩︎
- “Assuring Medicaid Beneficiaries Access to Hepatitis C (HCV) Drugs,” op. cit. ↩︎
- 42 USC § 1396r-8(d). States may restrict certain drugs categorically, but hepatitis C agents are not on this list. Statute allows states to restrict weight loss or weight gain drugs; fertility drugs; cosmetic or hair growth drugs; cold or cough relief drugs; smoking cessation drugs; vitamins and minerals, except for prenatal use; non-prescription drugs, except for pregnant women; drugs where along with them, the manufacturer requires certain tests or services be provided by a designee; barbituates; benzodiazepines; and drugs for sexual or erectile dysfunction, unless these drugs are used for another approved condition. ↩︎
- B.E. and A.R. v. Teeter, op. cit. and CMS, “Assuring Medicaid Beneficiaries Access to Hepatitis C (HCV) Drugs,” op. cit. ↩︎
- Methadone is a type of opioid. It is used for the treatment of opioid use disorder, and more recently, for the treatment of pain. However, it is a “complex medication to prescribe for pain relief,” and as a treatment for pain is associated with a disproportionate share of opioid-related deaths. See Wachino, 2016, op. cit. ↩︎
- Drug Utilization Reviews are a mechanism for state Medicaid programs to monitor beneficiaries’ prescriptions to prevent potential oversights that might harm beneficiaries, such as drug-disease contraindications or incorrect dosages. They are also a way for state Medicaid programs to identify overuse, abuse, or fraud within the prescription drug benefit. See “Drug Utilization Review,” CMS, accessed July 6, 2016, https://www.medicaid.gov/medicaid-chip-program-information/by-topics/benefits/prescription-drugs/drug-utilization-review.html. ↩︎
- Wachino, 2016, op. cit. CMCS also outlines ways to increase the availability and administration of naloxone, a drug used to counteract opioid overdoses, as well as ways to expand treatments for opioid use disorder. ↩︎
- Barry Meier and Sabrina Tavernise, “States Move to Control How Painkillers Are Prescribed,” New York Times, March 11, 2016, http://www.nytimes.com/2016/03/12/business/states-move-to-control-how-painkillers-are-prescribed.html ↩︎
- Ibid. ↩︎
- HHS, HHS awards $94 million to health centers to help treat the prescription opioid abuse and heroin epidemic in America, March 11, 2016, http://www.hhs.gov/about/news/2016/03/11/hhs-awards-94-million-to-health-centers.html. ↩︎
- “CDC Guideline for Prescribing Opioids for Chronic Pain.” CDC, accessed July 6, 2016, http://www.cdc.gov/drugoverdose/prescribing/guideline.html. ↩︎
- Vikki Wachino, “2016 Updates to the Child and Adult Core Health Care Quality Measurement Sets,” CMCS Informational Bulletin, CMS, December 11, 2015. https://www.medicaid.gov/federal-policy-guidance/downloads/cib-12-11-15.pdf. ↩︎
- “Drug Advertising: A Glossary of Terms,” FDA, accessed July 6, 2016, http://www.fda.gov/Drugs/ResourcesForYou/Consumers/PrescriptionDrugAdvertising/ucm072025.htm. ↩︎
- Wachino, 2015, op. cit. ↩︎
- Copyright 2016, Wolters Kluwer Clinical Drug Information, Inc. ↩︎
- “CDER List of Licensed Biological Products,” FDA, Center for Drug Evaluation and Research, accessed July 6, 2016, http://www.fda.gov/Drugs/DevelopmentApprovalProcess/ HowDrugsareDevelopedandApproved/ApprovalApplications/TherapeuticBiologicApplications/Biosimilars/ucm411418.htm “CBER List of Licensed Biological Products” FDA, Center for Biologic Evaluation and Research, accessed July 6, 2016, http://www.fda.gov/Drugs/DevelopmentApprovalProcess/ HowDrugsareDevelopedandApproved/ApprovalApplications/TherapeuticBiologicApplications/Biosimilars/ucm411418.htm. ↩︎
- Searchable version of the Orphan Drug Product designation database, FDA, http://www.accessdata.fda.gov/scripts/opdlisting/oopd/. ↩︎
- We pulled formulary information from CVS CareMark, Express Scripts, and Optum Rx. ↩︎
- “States Collection of Medicaid Rebates for Physician Administered Drugs,” (Washington DC: U.S. Department of Health and Human Services- Office of Inspector General, June 2011), http://oig.hhs.gov/oei/reports/oei-03-09-00410.pdf. For further discussion, see Bruen and Young, December 2014, op cit. ↩︎