How State Medicaid Programs are Managing Prescription Drug Costs: Results from a State Medicaid Pharmacy Survey for State Fiscal Years 2019 and 2020
Cost Containment and Utilization Control Strategies
How are states managing use and costs in their programs?
Managing the Medicaid prescription drug benefit and pharmacy expenditures remains a policy priority for state Medicaid programs, and state policymakers remain concerned about Medicaid prescription drug spending growth. Because state Medicaid programs are required to cover all drugs from manufacturers that have entered into a federal rebate agreement (in both managed care and FFS settings), states cannot limit the scope of covered drugs to control drug costs. Instead, states use an array of payment strategies and utilization controls, discussed below, to manage pharmacy expenditures.
Preferred Drug Lists (PDLs)
Most state Medicaid programs maintain a preferred drug list (PDL), a list of outpatient prescription drugs the state encourages providers to prescribe over others. A state may require prior authorization for a drug not on a preferred drug list or attach a higher copayment, creating incentives for a provider to prescribe a drug on the PDL when possible. In this way, a PDL allows a state to drive utilization to lower-cost drugs, including drugs for which the state has negotiated a supplemental rebate with the manufacturer. With the exception of four states (Hawaii, New Jersey, New Mexico, and South Dakota), all other responding states (46 of 50 states) reported having a PDL in place for FFS prescriptions as of July 1, 2019, and also negotiating supplemental rebates for preferred agents.
Most state P&T committees are responsible for determining PDL placement for new drugs (29 states). A small number of states reported that either the Medicaid agency (7 states) or the DUR board (6 states) is responsible for the review of new drugs for inclusion on the PDL.
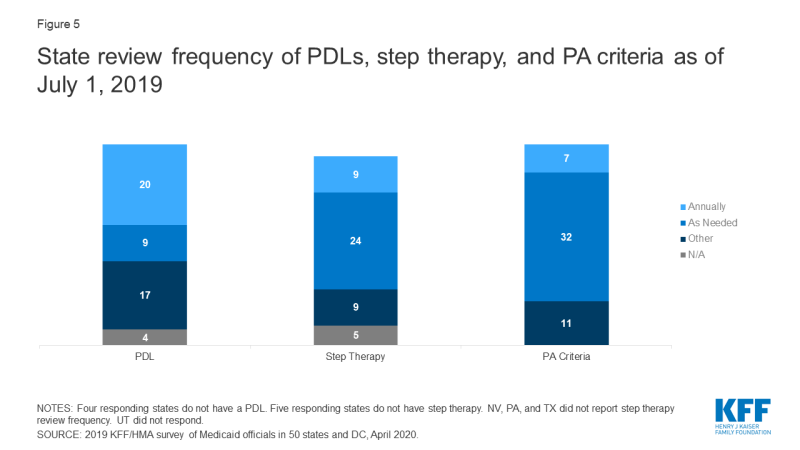
Most state DUR Boards and/or P&T Committees review PDLs at least annually. For PDL reviews, 20 of 50 responding states reported annual reviews, and 10 of the 17 states that reported other review periods review their PDLs quarterly, with an additional three states reviewing bi-annually (Appendix Table 5).
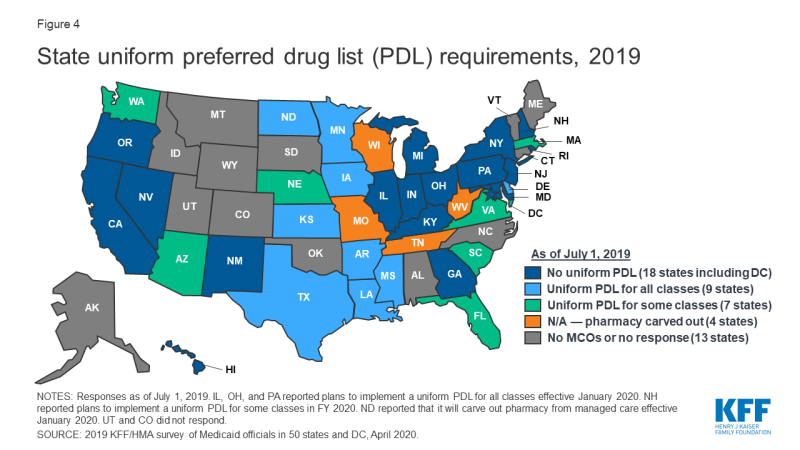
In recent years, a growing number of MCO states have adopted uniform PDLs requiring all MCOs to cover the same drugs as the state. In a previous survey of state Medicaid programs, just nine states reported having a uniform PDL across FFS and managed care in FY 2015.1 In this survey, 16 MCO states reported having a uniform PDL for some or all classes as of July 2019, 18 states reported no uniform PDL requirement (Figure 4). Eight states, however, reported plans to establish (Illinois, Ohio, New Hampshire, and Pennsylvania) or expand (Arizona, Massachusetts, Nebraska, and Washington) a uniform PDL in FY 2020. Several states noted that possible future changes were under consideration. Notably, Washington is considering including the physician-administered drugs on the PDL.
Though not all states utilize a uniform PDL, many states do review and approve MCOs’ PDL changes. More than half of the MCO states reported that the DUR Board, P&T Committee, or other state entity reviews and/or approves MCO PDL changes.
Prior Authorization and Step Therapy
States varied in what entity is responsible for developing prior authorization (PA) or step therapy criteria. One of the primary tools that almost all states have long used to manage drug utilization is prior authorization (PA), which requires prescribers to obtain approval from the state Medicaid agency (or its contractor) before a particular drug can be dispensed. States may also use step therapy, a type of PA for drugs that requires beneficiaries to begin treatment for a medical condition with a specific drug therapy, usually a lower-cost drug or generic, and progress to other therapies only if medically necessary. Sixteen states reported that the Medicaid agency is the entity responsible for setting PA criteria while 15 states reported that the DUR board sets PA criteria and the remaining states reported that the P&T committee (9 states) or other entities (9 states) fill this role (Table 3). Fourteen states reported the Medicaid agency develops step therapy criteria and 14 states reported that the DUR Board fills this role. The remainder of states reported utilizing either the P&T committee (12 states) or other entities (5 states).
Most states review PA and step therapy criteria on a less frequent basis than PDLs. The majority of states reported that both step therapy (24 states) and PA criteria (32 states) are reviewed “as needed” (Figure 5 and Appendix Table 5). The most common response for other review frequencies for both step therapy and PA criteria was quarterly.
Most states report that new drugs are subject to PA before undergoing initial review by the DUR Board and/or P&T committee. Four states without a PDL (Hawaii, New Jersey, New Mexico, and South Dakota) reported no PA requirement for new drugs while all other responding states reported that PA was always (16 states) or sometimes (30 states) required. For states answering “sometimes,” states identified reasons related to the new drug’s PDL status, cost and class as conditions for PA prior to review (Table 4). As one state noted, not requiring PA for a new drug could have a detrimental impact on supplemental rebates earned on existing PDL-preferred agents.
| Table 4: New Drug PA Reasons*, July 1, 2019 | ||
| Condition for PA | # of States | States (30 States Responding ) |
| Drug is in a current PDL class | 12 | AL, DC, DE, IN, LA, MT, NY, OH, PA, TN, VA, WV, |
| Cost threshold | 6 | DC, DE, MD, ND, NV, WV |
| Temporary PA required until full DUR BOARD and/or P&T Committee review | 5 | AK, FL, KS, OR, RI |
| PA always required except for protected drug classes (oncology, HIV, etc.) | 4 | CA, MI, MO, OR |
| Drug is in a PDL/therapeutic class that requires PA | 4 | DE, NC, TX, WY |
| Other^ | 6 | CO, MD, NE, OK, OR, WI |
| NOTES: *Responses were collected for the 30 states that reported not automatically subjecting all drugs to PA before initial review. States may have reported more than one condition for PA. ^Other reasons include statutorily required PA time limits, the drug not falling within a PDL class, the drug label having restrictive criteria, and other reasons as determined by the Medicaid agency. SOURCE: 2019 KFF and Health Management Associates (HMA) survey of Medicaid officials in 50 states and DC, April 2020. |
||
While states often impose PA on high-cost specialty or non-preferred drugs, a number of states have legislation protecting drug classes or categories from the use of these tools in some or all circumstances. Nearly half of the responding states (24) reported one or more state statutory limits in place on the Medicaid agency’s ability to subject a drug or drug class to FFS utilization controls such as PA or step therapy requirements (Table 5). In some cases, states noted that the statutory prohibition applied to PDL placement but did not prevent the Medicaid agency from imposing quantity limits. Of the 24 states with a statutory limit on utilization controls, 17 included pharmacy as a covered benefit under an MCO arrangement. All but five of these states (Maryland, Michigan, North Dakota, Rhode Island, and Virginia), also applied the statutory limits to MCOs.2
| Table 5: State Statutory Limits on Utilization Controls, July 1, 2019 | ||
| Drugs/Drug Classes Protected | # of States | States (50 States Responding) |
| HIV/AIDS antiretrovirals | 14 | AL, CO, CT, FL, LA, MD, MI, ND, NV, OK, RI, TX, VA, VT |
| Mental health drugs | 10 | CO, CT, HI, IN, MI, MO, ND, OH, OR, VA |
| Cancer drugs | 6 | CO, MI, ND, TN, TX, VA |
| Epilepsy drugs | 4 | CO, CT, IL, MI |
| Medication Assisted Treatment | 3 | DC, IL, TX |
| Other* | 6 | KS, MI, NC, NV, RI, VA |
| NOTES: *Other drugs reported include antihemophilic medications, organ transplant drugs, multiple sclerosis drugs, brain disorder drugs, therapeutic classes with only one drug, or drugs with very low utilization. SOURCE: 2019 KFF and Health Management Associates (HMA) survey of Medicaid officials in 50 states and DC, April 2020. |
||
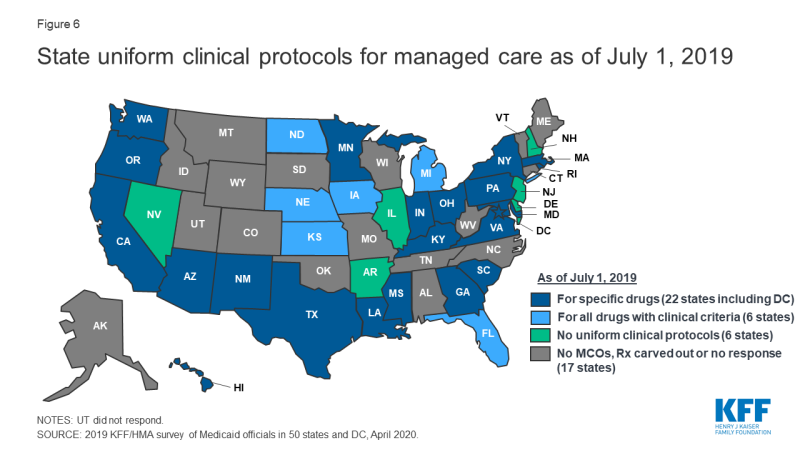
States are active in their management of MCO clinical protocols, or medical necessity criteria, with 28 states reporting that they require uniform clinical protocols for some or all drugs with clinical criteria. Only six states reported no uniform clinical criteria (Figure 6). In addition, five states report that they plan to impose uniform protocols on at least one drug in 2020 (Arizona, Louisiana, Ohio, Pennsylvania, and Washington). No states reported plans to remove any uniform clinical protocols.
Most states that use MCOs to deliver pharmacy benefits require plans’ PA policies to be no more restrictive than or the same as state rules in FFS. Of the 34 responding states3 that included pharmacy as a covered benefit under an MCO arrangement as of July 1, 2019, nearly two-thirds of these states (22) required MCO policies to be no more restrictive than FFS policies (Table 6).
| Table 6: Limits on MCO PA and Step Therapy Criteria, July 1, 2019 | ||
| Drug/Drug Classes Protected | # of States | States (34 MCO States Responding) |
| Required to be no more restrictive | 22 | AR, CO, DC, DE, FL, GA, IL, KY, LA, MA, MS, ND, NE, NH, NY, OH, OR, PA, RI, TX, VA, WA |
| Varies | 4 | HI, MN, NJ, SC |
| Required to be the same | 2 | IA, KS |
| Required to be no less restrictive | 2 | NM, NV |
| Permitted to be more restrictive | 2 | CA, MD |
| Other | 2 | IN*, MI+ |
| NOTES: *IN reported that MCO contracts requirements are being transitioned to require “no more restrictive” policies as contracts come up for renewal. +MI reported that MCO policies can only be less restrictive than the PA and step therapy criteria outlined in the MCO common formulary. SOURCE: 2019 KFF and Health Management Associates (HMA) survey of Medicaid officials in 50 states and DC, April 2020. |
||
Similar to their oversight of PDL changes, some states report oversight of MCO PA and step therapy criteria regardless of their uniform requirements for these rules, with the majority of those responding indicating that the state oversees either step therapy, PA/utilization management criteria, or both.
Prescription Limits
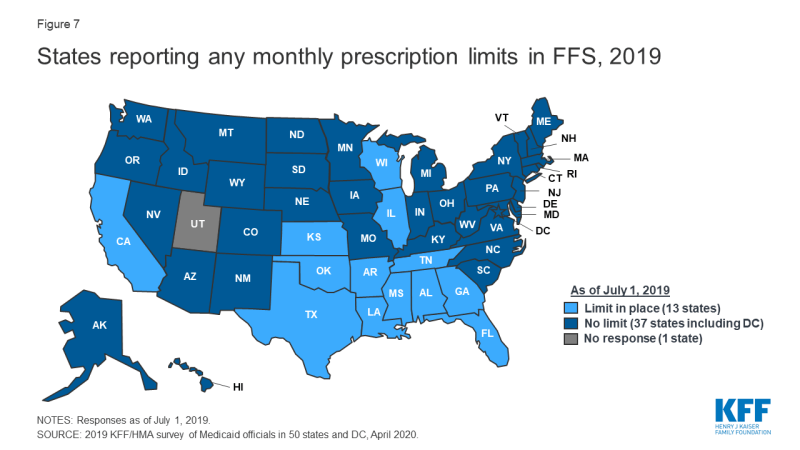
Some states report imposing limits on the number of total and/or brand prescriptions a beneficiary may access in a month without PA. States may limit the number of prescriptions a beneficiary may access without prior authorization,4 but prescribers and pharmacists may submit requests to override these limits when medically necessary or under other specific circumstances. About one-quarter of the 50 responding states5 (13 states) reported imposing a monthly limit on FFS prescriptions (Figure 7 and Appendix Table 6). Nine of these 13 states reported that the limit could be overridden using PA or other appeals process.6 Three states reported applying monthly prescription limits only to narcotics and seven of the remaining 10 states with limits noted a number of excluded drugs or drug classes such as HIV antiretrovirals (6 states), cancer drugs (5 states), family planning products (5 states), mental health drugs (4 states), and tobacco cessation products (3 states). Six of the 13 states reported applying the limits only to adults, and four states noted specific exemptions for persons receiving long term services and supports.
Prescription limits were less likely to be uniform across FFS and MCOs as other utilization management tools. States imposing monthly limits were also asked to indicate if MCOs were required to apply the same limits, PA/appeals processes, and exemptions. Four of the 13 states had no MCOs (Alabama and Oklahoma) or did not include pharmacy as an MCO-covered benefit (Tennessee and Wisconsin). Of the remaining nine states, only one (Mississippi) required MCOs to apply the same limits and two states (Florida and Louisiana) noted that MCOs could be less restrictive, but not more restrictive, than the state’s FFS policy. Only one state reported a planned change for FY 2020: Mississippi increased the number of drugs covered in its monthly prescription limit to six from five effective July 1, 2019.
Generic Drug Policies
Almost all states have policies or tools in place to promote generic utilization. As shown in Table 7 and Appendix Table 7, the most common policy reported was a mandatory generics policy (41 states). Only three states (California, New Hampshire, and Nevada) reported having no policies or tools in place. No state reported planned changes to their policies for generic utilization for FY 2020, although one state noted that where applicable, the state will switch from a preferred brand to a preferred generic if the generic becomes less expensive.
| Table 7: Policies/Tools in Place to Promote Generic Utilization, July 1, 2019 (50 states responding) |
|
| Policy/Tool | # of States |
| Mandatory generics | 41 |
| Lower copayment requirement for generics | 17 |
| Provider education | 13 |
| PDL placement | 5 |
| Higher point of sale dispensing fee for generic substitution | 3 |
| Tiered dispensing fee based on pharmacy’s generic drug utilization rate | 1 |
| Other* | 3 |
| No policies or tools | 3 |
| NOTES: *Other policies and tools reported were a requirement to cover drugs with the lowest net costs, which could be either a brand or a generic (ID and ME), and a lower required copayment for selected generics treating specific conditions (MA). SOURCE: 2019 KFF and Health Management Associates (HMA) survey of Medicaid officials in 50 states and DC, April 2020. |
|
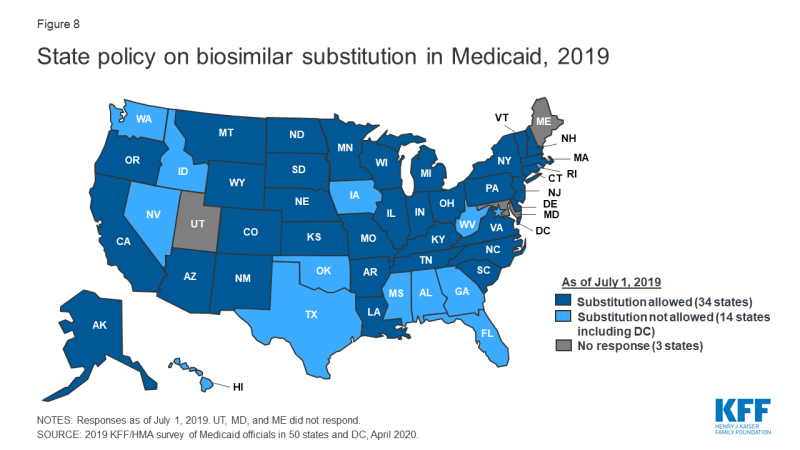
In contrast, states are less likely to require biosimilar substitution, though most allow it. No state reported requiring pharmacists to substitute a biosimilar for a prescribed biologic, but over two-thirds of responding states (34 of 48 states)7 indicated that biosimilar substitution was allowed (Figure 8).
The vast majority of responding states (45 of 50) require biosimilar drugs to undergo the same DUR Board/P&T committee review process as other drugs. Five states, however, reported a different process: Arizona’s P&T Committee reviews biosimilars only if the branded product is available within 180 days of when the biosimilar becomes available in the market; Montana will use the same criteria as the original product, unless the DUR Board requests a separate review; New Mexico reported covering biosimilars based on FDA approval and a CMS rebate agreement; Rhode Island indicated that the Medicaid agency reviews biosimilars; and Vermont reported performing a financial analysis in place of a full new drug review.
About half of MCO states align some or all FFS and MCO generic substitution policies. Of the 34 responding states that include pharmacy as an MCO covered benefit, 12 reported that MCOs were required to follow the state’s FFS generic policies,8 while three MCO states (Minnesota, New York, and Washington) reported that MCOs were required to following MCO policies in part.