
The independent source for health policy research, polling, and news.
New KFF Analysis Examines Rapidly Evolving Federal Policies For Substance Use Disorder Treatment for the Opioid Epidemic
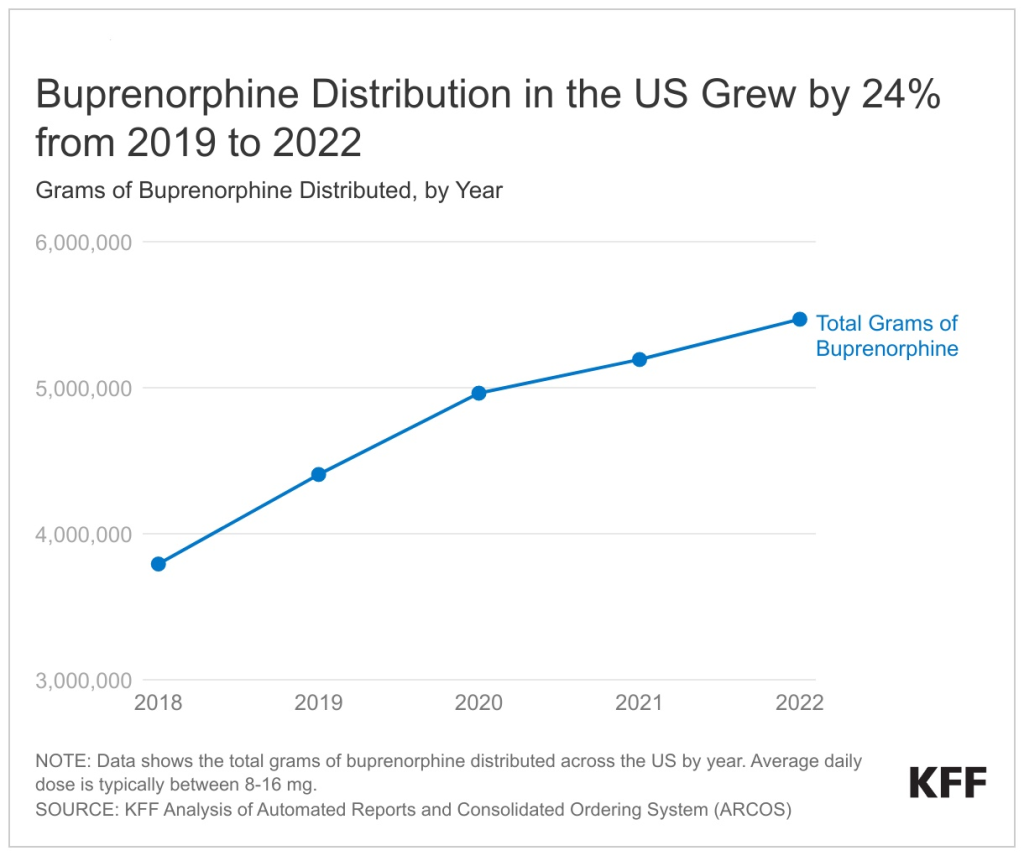
A new KFF analysis finds that 24 percent more buprenorphine, a medication to treat opioid use disorder, was dispensed in 2022 than in 2019, the year before the pandemic brought a surge of opioid overdose deaths – and a focus on how to expand access and treatment.
This upward trend in buprenorphine distribution, already in motion before the pandemic, continued throughout the COVID public health crisis, suggesting continued improvements in access to treatment even as the pandemic raised other barriers to health care. However, it is unclear whether these steps to improve access to buprenorphine are reaching people with high needs, including communities of color.
KFF examined five key federal policies governing substance use disorder treatment, the changes they have undergone during the pandemic, and the implications for access and treatment for opioid use disorder. Data show a steep increase in opioid overdose deaths during the pandemic, primarily driven by the synthetic opioid fentanyl. From 2016 to 2021, opioid overdose deaths nearly doubled, from 42,249 to 80,411. The increase has been particularly high among people of color and young people.
The key findings include:
- Policy changes during the pandemic increased access to care by making it possible to initiate buprenorphine treatment via telehealth, without an in-person visit. Although federal officials have considered ending that flexibility, it has been extended temporarily in response to public concerns over the likely impact on access to treatment.
- Late last year, federal legislation removed the additional barriers to buprenorphine prescribing for OUD treatment, the so-called X-waiver, opening the doors to a substantial increase in authorized providers. But research indicates that many prescribers still may not prescribe buprenorphine and substantial disparities in access to treatment may remain.
- A temporary pandemic-era policy has allowed opioid treatment programs to provide some patients with up to 28 days of take-home doses of methadone, a change that may become permanent under a proposed rule. This shift was designed to ease the burden for patients and increase access to treatment for those living farther from treatment centers.
- Recent Food and Drug Administration approval of over-the-counter naloxone – a nasal spray to reverse opioid overdose – allows the life-saving drug to be purchased without a prescription, though its roughly $50 price may remain a barrier. Accessibility of fentanyl test strips, which can help users determine if drugs have been mixed with fentanyl, remains limited, though federal funds can now be used to pay for them under certain grant programs.
- As of June 2023, 14 states have submitted Section 1115 waivers seeking exemption from federal law that prohibits the use of federal Medicaid dollars for health care services for inmates, including opioid use disorder treatment. Nearly two-thirds of prison inmates have a substance use disorder, and their risk of opioid overdose after being released is 10 times higher compared to the general public.
Though new and proposed federal policies have the potential to increase access to care, ongoing challenges, such as behavioral health workforce shortages, low prescribing of buprenorphine by providers, and treatment gaps by race/ethnicity, could limit the effectiveness of new federal strategies.
For more data and analyses about the opioid epidemic, visit kff.org.
Related Resources: