Novel Coronavirus “COVID-19”: Special Considerations for Pregnant Women
|
Key Takeaways |
|
Introduction
The novel coronavirus, also known as “SARS-CoV-2” causing the illness “COVID-19”, has sparked international concern and emergency response. While both men and women are affected by COVID-19, this brief outlines considerations for how the pandemic may specifically impact pregnant women. With over 6 million pregnancies per year in the U.S., pregnant and breastfeeding women constitute a significant portion of the population that could be impacted by COVID-19. This brief summarizes what is known thus far about pregnancy and COVID-19.
What do we know thus far about the impact of COVID-19 in pregnancy?
Does risk for COVID-19 differ between men and women?
The COVID-19 outbreak is an evolving pandemic. Little is known on how, or if, the disease differentially impacts women compared to men. To date, initial studies on the outbreak in China have found men may account for slightly more of the overall cases, and that men may have a slightly higher mortality rate from COVID-19. This could be due to biological factors (i.e. differences in immune response), medical factors (i.e. comorbidities) and lifestyle factors (i.e. smoking). However, most trackers of the pandemic, including the CDC and WHO, have not published their data by gender. Therefore, more research on this topic is warranted before conclusions are made.
Does risk for COVID-19 differ between pregnant and non-pregnant women?
According to the CDC, there is insufficient data at this time to know whether pregnant women are at increased risk for adverse health outcomes if infected by the novel coronavirus as compared to non-pregnant people. A WHO-China Joint Mission investigation of 147 pregnant women in China with suspected or confirmed COVID-19 found that 8% had severe disease and 1% were in critical condition (14% severe, 6% critical for the overall population). In a small study of pregnant women in Wuhan, China, the clinical characteristics and severity of COVID-19 also appeared similar between pregnant and non-pregnant women. That said, the American College of Obstetricians and Gynecologists (ACOG) issued a statement that “pregnant women may be at higher risk of severe illness, morbidity, or mortality compared with the general population,” likely due to physiologic changes that happen during pregnancy, and because pregnancy constitutes a state of relative immunosuppression as compared to non-pregnancy.
Can the novel coronavirus be transmitted during pregnancy or breastfeeding?
Data are also lacking about whether pregnant women infected by the novel coronavirus can pass it to their fetuses across the placenta during pregnancy, called “vertical transmission.” However, several small studies of pregnant women infected with the novel coronavirus found no evidence of vertical transmission, as none of their infants tested positive at birth, and the virus was not detected in samples of the amniotic fluid, umbilical cord blood or placental tissue (Zhu et al. 2020; Chen et al. 2020; Chen et al. 2020; Zhang et al. 2020; Li et al. 2020). That said, a few cases of newborns infected by the novel coronavirus have been reported, and it remains unclear if they were infected before, during or after delivery (Qiao, 2020; Murphy, 2020). There is no evidence to date to suggest the novel coronavirus can pass to infants through breastmilk, however the CDC has issued precautionary guidance for women with suspected or confirmed COVID-19 who are also breastfeeding.
Adverse health outcomes have been found in infants born to mothers affected by COVID-19, including respiratory distress, premature labor, and even death. However, it is unclear whether these adverse outcomes are related or not to the COVID-19 infection in their mothers. Meanwhile, guidance published by the Royal College of Obstetricians and Gynecologists (RCOG) suggests there is no data yet linking COVID-19 with an increased risk of pregnancy loss. As for maternal outcomes, some initial evidence indicates outcomes are similar between women with and without COVID-19, however other studies show symptom severity in pregnancy varies from asymptomatic to life-threatening. As the outbreak continues, more data on maternal and neonatal outcomes will likely come forward.
Access to Care
Will pregnant women be reluctant to access prenatal care due to fear of COVID-19 exposure in medical settings?
Much of the general public is worried about COVID-19. A recent KFF poll conducted from March 11-15 found 62% of adults reported being very or somewhat worried that they or someone in their family will get sick from the coronavirus. 51% of adults reported being very or somewhat worried about putting themselves at risk of exposure to the virus because they can’t afford to stay home and miss work.
For pregnant women, concern over COVID-19 may be even more heightened.Social distancing, which is now recommended as a response to the containing the spread of coronavirus transmission, presents distinct challenges for pregnant women. This advice may be hard to follow in pregnancy; most women have monthly to weekly interactions with the health system during pregnancy for prenatal checkups.
Can telemedicine be used to provide more services to pregnant women?
One possible way to provide access to prenatal care during this outbreak is to expand use of telemedicine during pregnancy; this would enable some pregnant women to stay home and participate in prenatal visits over videoconference or the phone, without coming into clinic where they risk COVID-19 exposure (Figure 1). A KFF brief explains more about potential uses of telemedicine in pregnancy.
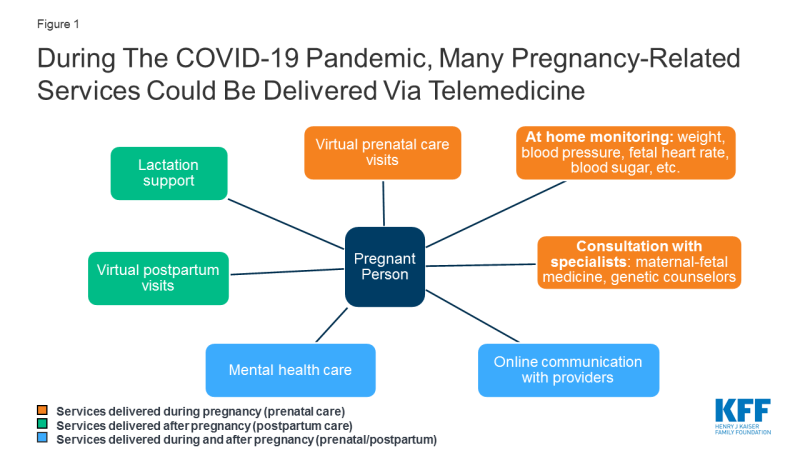
Figure 1: During The COVID-19 Pandemic, Many Pregnancy-Related Services Could Be Delivered Via Telemedicine
Currently, however, utilization of telemedicine for pregnancy-related services is minimal. Lack of insurance coverage for telemedicine poses a large barrier to its implementation. Nearly half of all births in the U.S. are financed by Medicaid, but only a handful of state Medicaid programs specifically address obstetrical care in their telemedicine reimbursement laws. No states specifically require private insurance plans to cover pregnancy services in their telemedicine reimbursement laws. However, in approximately half of states, if telemedicine services are shown to be medically necessary and meet the same standards of care as in-person services, private insurance plans must cover telemedicine services if they would normally cover the service in-person.
Several major health insurance companies have mentioned coverage of telehealth in their response to COVID-19. For example, Aetna is offering telemedicine visits for any reason without copays, while Humana is waiving telemedicine costs for urgent care visits for 90 days. Others are “encouraging” use of telehealth, without word on cost sharing. In a White House Coronavirus Task Force briefing, Vice President Pence referenced the recently enacted Coronavirus Preparedness and Response Supplemental Appropriations Act, which broadens coverage and reimbursement for telemedicine services for Medicare. He also mentioned Medicaid may similarly make these services available. That said, the details of telehealth coverage remain murky, and there are likely to be significant gaps in access to telemedicine during this pandemic. Even beyond coverage considerations, there are logistical challenges that come with implementing a telemedicine program at a health center and start-up costs are high. For example, telemedicine platforms must be compliant with the Health Insurance Portability and Accountability Act (HIPAA), and integrate into an existing electronic health record, and clinicians must ensure their malpractice insurance covers telemedicine.
Even if telemedicine implementation became more widespread, almost all births in the U.S. occur in hospitals. Shortages of personal protective equipment (PPE) like masks, gloves, and gowns, and other hospital resources have been predicted by the World Health Organization as a result of the COVID-19 pandemic which could make frontline health workers increasingly vulnerable to COVID-19. In turn, patients may fear exposure to the virus while in the hospital for labor and delivery. Some women may also need to rearrange their birth plans, if family can no longer travel to be with them in the hospital, if family members are in quarantine, or if spouses have to stay home with children due to school closures.
Drug Development and Payment Policy
Will COVID-19 treatment and vaccine development for pregnant women lag behind rest of the population?
Implementation of an effective vaccine for the novel coronavirus is likely months to years in the future, however it is important to understand how the drug development process impacts pregnant women and how that could affect the use of any future COVID-19 treatment or vaccine. For almost all drugs in development, pregnant women (and often women who are breastfeeding) are specifically excluded from drug development trials. This is done to avoid exposing a fetus or breastfed infant to potentially harmful drugs. However, this means that when most drugs obtain FDA approval, they have not been tested in pregnant and lactating women. Only in post-approval studies, if ever, is the drug tested for these populations. This presents a lag time between when a drug is approved for use in the non-pregnant population and when safety and efficacy data is available for pregnant persons.
This same delay will likely hold true in the development of COVID-19 treatments and vaccines. To use another infectious disease as an example, the ministry of health in the Democratic Republic of Congo administered the Ebola vaccine to non-pregnant people months in advance of pregnant women; meanwhile, 319 pregnant women and 603 lactating women with infected contacts were excluded from vaccination from November 2018 to May 2019, even after the vaccine was approved for use in pregnant/lactating women in February 2019. During the 2009 H1N1 “swine flu” epidemic, pregnant women were especially vulnerable to severe complications. Vaccination of pregnant women was made a priority by the CDC and WHO, but even still, many pregnant people experienced delays in vaccination.
While there are often very compelling reasons to exclude pregnant women from research studies, it is significant to note potentially lifesaving COVID-19 treatment and vaccines may never be tested or approved for pregnant or lactating women, unless dedicated efforts to do so are made. It will be important that research and development on new treatments and vaccines for COVID-19 address the inclusion and needs of pregnant and lactating women in upcoming clinical trials. Of note, a current clinical trial of a possible COVID-19 treatment does not allow pregnant or breastfeeding women to participate at this time.
How will cost impact pregnant women’s access to COVID-19 treatments and vaccine once available?
When treatment and vaccines are eventually developed for COVID-19, cost sharing could hinder access to care. Cost plays a large factor into care seeking behavior. While women and men both feel the impact of health costs, data from KFF’s 2017 Women’s Health Survey show that cost can be particularly burdensome for women, who on average earn lower wages, have fewer financial assets, accumulate less wealth, and have higher rates of poverty than men. For example, 19% of women reported they put off or postponed preventative services in the last year due to cost, while 26% of women delayed or went without care due to cost.
The ACA requires most private health insurance plans and states with Medicaid expansion to cover recommended preventive services without cost sharing, including vaccines recommended by the Advisory Committee on Immunization Practices (ACIP). Once ACIP recommends a new vaccine, however, it typically takes another year for coverage to commence (or in the next plan year). Therefore, even once a COVID-19 vaccine is developed and recommended by ACIP, there will likely be a significant lag time between when the vaccine is required to be covered without cost sharing. A few health insurers have said so far that they will offer a coronavirus vaccine without cost sharing once developed. CMS says a vaccine will be covered by Medicare Part D. For the individual and small group markets, and states with Medicaid expansion, the vaccine would likely be covered if and when it becomes a recommended preventive service by ACIP.
Research shows us that many pregnant women forgo recommended vaccines if not provided free of cost. A study of pregnant Medicaid patients in Florida found that vaccination rates for Tdap and influenza were low in the prenatal period when Medicaid did not cover these vaccines. However, rates rose in the postpartum period, when the vaccine was covered free of charge. These data suggest that costs pose a barrier to vaccination for at least a portion of pregnant patients.
Conclusions
There are a number of specific reasons why pregnant women may be uniquely affected by the COVID-19 pandemic, but information is limited currently. There is no definitive answer at this time about if the virus is transmitted during pregnancy or in breastmilk and more research is warranted about potential adverse health outcomes to mothers and infants. Inclusion of pregnant women and lactating women in treatment and vaccine development for COVID-19 will be important, as access to novel therapies for these groups has historically lagged far behind the non-pregnant population. Use of telemedicine for prenatal care visits could help pregnant women reduce their risk of virus exposure, however most pregnant women will still need to be admitted to hospitals for labor and delivery, potentially at time when hospitals are stressed beyond their capacity and resources. Keeping in mind the pregnant population during the COVID-19 pandemic may help mitigate potential preventable health disparities.
