A Look at How Medicaid Agencies Are Assisting with the COVID-19 Vaccine Roll-Out
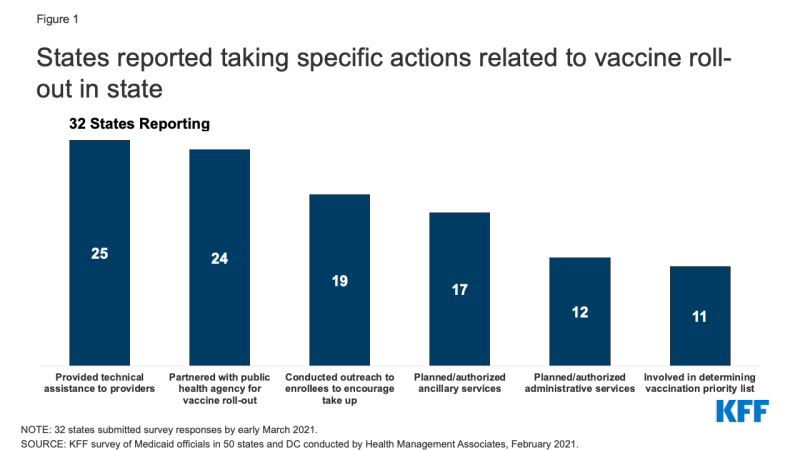
As of February 2021, there are three COVID-19 vaccines approved for emergency use in the U.S. and states are playing a central role in their timely distribution and prioritizing eligible populations. Because Medicaid covers for over 77 million enrollees, including groups disproportionately at risk of contracting COVID-19 as well as hard-to-reach groups, Medicaid agencies can be important partners for the public health agencies that are typically leading the state COVID-19 vaccination efforts. In mid-February 2021, the Kaiser Family Foundation (KFF) and Health Management Associates (HMA) fielded a two-part rapid, mini-survey of Medicaid directors in all 50 states and the District of Columbia. Of the 32 states that responded to Part II of the survey, 29 reported that Medicaid agencies were involved in at least one vaccine roll-out activity (Figure 1). This brief provides insights regarding how state Medicaid agencies are assisting with the COVID-19 vaccine roll-out including specific actions taken. (See separate brief for findings from Part I of the mini-survey related to Medicaid enrollment and spending trends.)
Three-quarters of responding states (24 of 32) reported partnering with public health or other agencies to plan and implement the vaccine roll-out. Several states indicated close coordination with their state departments of public health that are taking the lead on vaccine distribution and roll-out. For example, one state commented that it amplifies the public health department’s messaging and communications, but also periodically relays back concerns from key Medicaid stakeholders (e.g., behavioral providers not included in the Phase 1a priority group for health care workers). Fewer states, however, reported being involved with determining state vaccination priority lists (11 of 32).
Over three-quarters of responding states indicated their state Medicaid agencies are providing technical assistance to providers (25 of 32). States reported communicating directly with Medicaid providers regarding the roll-out of COVID-19 vaccinations through, for example, provider bulletins and dedicated webpages that include information on COVID-19 vaccination coverage, reimbursement, and billing and a dedicated email address/inbox for provider questions. One state also reported that it has worked to ensure that there is an expedited process for vaccinators to enroll in Medicaid (if not already enrolled).
Over half of responding states reported conducting outreach to Medicaid enrollees to encourage COVID vaccination take-up (19 of 32). Several states discussed working with Medicaid managed care plans on vaccine roll-out activities including: messaging to address member vaccine hesitancy; clarifying the availability of MCO care coordination and transportation services that could reduce vaccine access barriers; coordinating COVID vaccine appointments; calling high-risk members, and making vaccination appointment reminder calls. Over half of responding states (17 of 32) are expanding services to assist with vaccine roll-out. For example, a few states specifically mentioned authorizing nonemergency medical transportation (NEMT) services for vaccine appointments. A number of states (12 of 32) also reported planning or authorizing new or expanded Medicaid administrative supports. One state, for example, commented that its existing nurse advice line will provide general information to callers regarding vaccine administration.
The majority of responding states reported that the Medicaid agency is involved with specific activities to assist with vaccine roll-out to groups disproportionately covered by Medicaid. About two-thirds of responding states (22 of 32) reported that their state Medicaid agencies are involved with specific activities to assist with vaccine roll-out to HCBS recipients. About half of responding states also reported assisting with vaccine roll-out efforts for residents of non-nursing home institutions, communities of color, and people in rural areas (Figure 2). Several states commented on a number of specific activities underway including:
- Data sharing with the public health agency on seniors receiving HCBS who are not in a nursing facility or alternative living facility. The public health agency matched the data provided against its vaccine registry and is now conducting outreach to those not yet vaccinated.
- Developing quality measures focused on closing vaccination disparities between whites and communities of color.
- Proactively outreaching to NEMT drivers to get vaccinated and drive to vaccination events.
- Requesting federal approval to provide COVID-19 vaccines to populations who are in limited benefit programs (e.g., family planning program, COVID-19 uninsured group, and restricted scope programs where only emergency and pregnancy related services are offered).
- Partnering with Rite Aid to provide vaccinations to long-term care facilities not covered by the federal pharmacy partnership program.
- Encouraging MCOs to utilize community health workers to conduct outreach to individuals that are transient or difficult to reach, including individuals experiencing homelessness, people with disabilities, or individuals with substance use disorders.
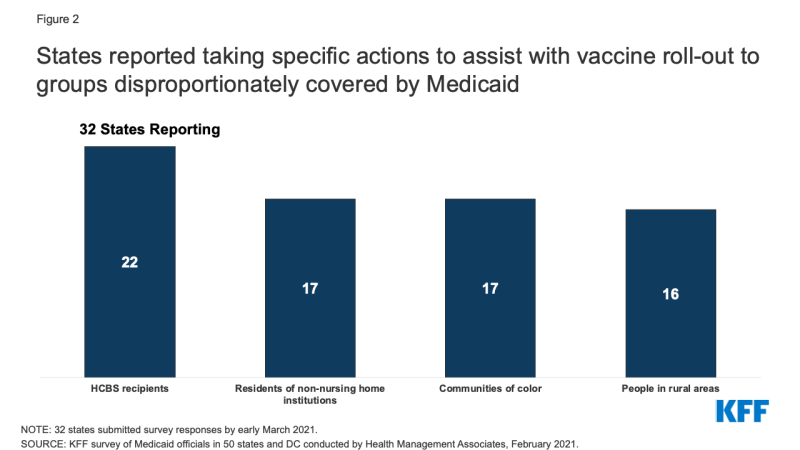
Figure 2: States reported taking specific actions to assist with vaccine roll-out to groups disproportionately covered by Medicaid
More than three-quarters of states (26 of 32) indicated at least one way in which partnerships between public health and Medicaid had been or could be further developed to assist with vaccine roll-out efforts. The most common areas of opportunity reported were coordinating outreach, data sharing, and holding daily or weekly meetings between agencies. States described other activities that could assist in vaccine roll-out efforts including: creating opportunities to have staff work across agencies; coordinating to assist individuals without internet access and individuals whose first language is not English in scheduling vaccination appointments, and working to help ensure and promote equity regarding the vaccine roll-out.