DMPA Contraceptive Injection: Use and Coverage
Note: Figure 1 was updated on Sept. 18, 2024, to correct a typographical error. The FPL for a family of three in 2017 was $19,730, not $19,370.
The DMPA contraceptive injection is a commonly used reversible contraceptive method among women in the United States. Also known as the “shot,” the injection is commonly known by its brand-name Depo Provera (depot-medroxyprogesterone acetate or DMPA), although generic alternatives are available. It was first introduced in the United States in 1959 for management of menstruation and was approved for contraceptive use by the U.S. Food and Drug Administration (FDA) in 1992. This factsheet provides an overview of the types, use, awareness, availability, and insurance coverage of contraceptive injection in the U.S.
How does the DMPA Injection work?
The shot works by releasing the hormone DMPA, a progestin, which suppresses ovulation and thickens cervical mucus that also helps keep sperm from fertilizing an egg. DMPA can be provided by an intramuscular shot (DMPA-IM) administered by a clinician or by a subcutaneous shot (DMPA-SC) that can be injected by the patient at home (Table 1). Both forms are FDA-approved and need to be administered once every 12 weeks to be effective. Brand-name Depo-Provera and the generic equivalents for medroxyprogesterone acetate are intramuscular injections, providing 150mg of progestin in one dose. The Depo-subQ Provera 104 injection uses a smaller needle and a lower dose of progestin (104 mg) than the intramuscular alternative. Because Depo-subQ Provera 104 uses a smaller needle, it can be less painful than the intramuscular injection and can be administered by the patient at home. This method is considered a safe, “off-label” method while having the same contraceptive effectiveness. Like most contraceptives, the DMPA shot does not protect against STDs; use of condoms is recommended to reduce the risk for STDs, including HIV, while using DMPA shots.
Table 1
Types of Birth Control Injections |
|||||
| Injection Method | Injection Name | Injection Administrator | Dosage | Injection Frequency | Possible Side Effects |
| Intramuscular | Brand: Depo-Provera
Generic alternatives are also available. |
Provider only. | 150mg of progestin. | 12 weeks (3 months). | Menstrual irregularities.
Weight gain. Bone density loss. |
| Subcutaneous | Brand: Depo-SubQ Provera 104
No generic alternative is available yet. |
Provider or patient. | 104mg of progestin. | ||
The DMPA shot has a typical use failure rate of 4% when used once every 12 weeks or three months. Long-acting reversible contraceptive (LARCs) methods such as implants, intrauterine devices (IUDs), and permanent contraceptive methods such as vasectomies and tubal ligations are typically more effective than the shot because these methods involve little to no follow-up care, while injections need to be repeated every 12 weeks in order to be effective. Condoms or another non-hormonal contraceptive are recommended as a back-up for 7 days after the first injection. If a patient is more than four weeks late for a shot (16 weeks after their last shot), it is recommended that they take a pregnancy test before the next dose and that they use condoms or another non-hormonal contraceptive as a back-up for another 7 days if they receive another shot. It takes an average of 10 months for pregnancy to occur after stopping the injection, which is comparable to other methods such as IUDs and oral contraceptive pills.
Benefits and Side Effects
The DMPA shot has several non-contraceptive benefits but also has some side effects and risks. Benefits include lower risk of uterine cancer and reduced symptoms of endometriosis. However, the injectable has the highest discontinuation rate among contraceptives in the U.S., associated with side effects which include menstrual irregularities (spotting or cessation of periods) and weight gain. Of note, Depo-Provera comes with a black box warning from the FDA that the contraceptive injection should not be used as a long-term (longer than 2 years) method unless other contraceptive methods are considered inadequate, as women who use Depo-Provera may lose significant bone density. However, the American College of Obstetricians and Gynecologists (ACOG) and the World Health Organization (WHO) disagree with this warning, stating that loss in bone density from DMPA is not associated with fractures and appears to be reversible after discontinuation of the injectable. Both organizations conclude that the benefits of DMPA use outweigh the theoretical fracture risk, and that DMPA may be prescribed without limitations on its duration of use.
Use, Awareness, and Availability of the Contraceptive Injection
Based on the most recent data on contraceptive use from the National Survey of Family Growth, approximately 3% of women who used contraception from 2017-2019 reported that they used the contraceptive injection in the past month. Over the last two decades, women’s access to and options of various contraceptive choices have changed, and overall use of the injection has declined, as more women are using LARCs, such as IUDs and implants. During the first few years of the COVID-19 pandemic, however, the availability of and interest in using DMPA-SC increased due to restrictions placed on in-person clinic appointments. A 2023 Contraception study reports that there was a significant increase in provision of DMPA-SC for self-injection during this time, although this is still a small portion of overall use of DMPA-SC.
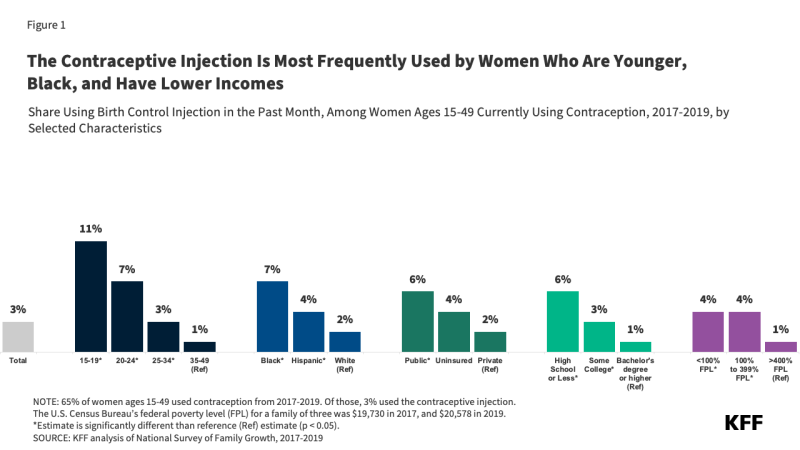
Among reproductive age women who used any form of contraception from 2017-2019, the contraceptive injection was most often used by young women, lower-income women, and Black women. Use of the injection also decreased with educational attainment – smaller shares of women with bachelor’s degrees reported using the shot as their contraceptive method (Figure 1).
Insurance Coverage and Cost of the Contraceptive Injection
The Affordable Care Act (ACA) requires most private insurance plans and Medicaid expansion programs to cover one of each of the 18 FDA-approved contraceptive methods without cost sharing. Women with private insurance and those with Medicaid coverage are eligible for patient education, counseling, and access to at least one form of the contraceptive injection without cost-sharing. Coverage also includes the first doctor’s visit, discontinuation of the shot, and management of side effects. Although coverage includes at least one form of the injection, plans may not cover both the intramuscular formulation and Depo-subQ Provera 104; however, if a clinician determines that a particular injectable formulation is medically appropriate for a patient then the plan must cover that form.
Safety-net clinics that participate in the federal Title X family planning program offer low or no-cost contraceptives to uninsured adults and teens on a sliding scale and may forego charges for those on the lowest end of the income scale. The Office of Population Affairs (OPA) within the U.S. Department of Health and Human Services (HHS) reported that, in 2022, approximately 302,000 women received the DMPA injection as their primary contraceptive method from a Title X service provider.
In 16 states (CA, CO, HI, ID, IL, IN, MD, ME, MN, NV, NM, NH, OR, SC, TN, VA) and the District of Columbia, pharmacists can provide the intramuscular contraceptive shot directly to women without the need to first visit a physician to obtain the prescription and injection (Figure 2). In some states like Hawaii, Indiana, and Maryland, the statewide protocol or standing order does not explicitly state that the DMPA injection can be offered but does state that all FDA-approved hormonal contraceptive methods can be administered by pharmacists. However, pharmacist participation in these programs is not required and implementation has varied across states. Furthermore, while the actual DMPA shot is typically covered, women will likely have to have to pay out-of-pocket for a pharmacist consultation fee, which is not required to be covered under the contraceptive coverage laws in most of these states and by federal law through the ACA.
