Infographic: The Availability and Use of Medication Abortion Care
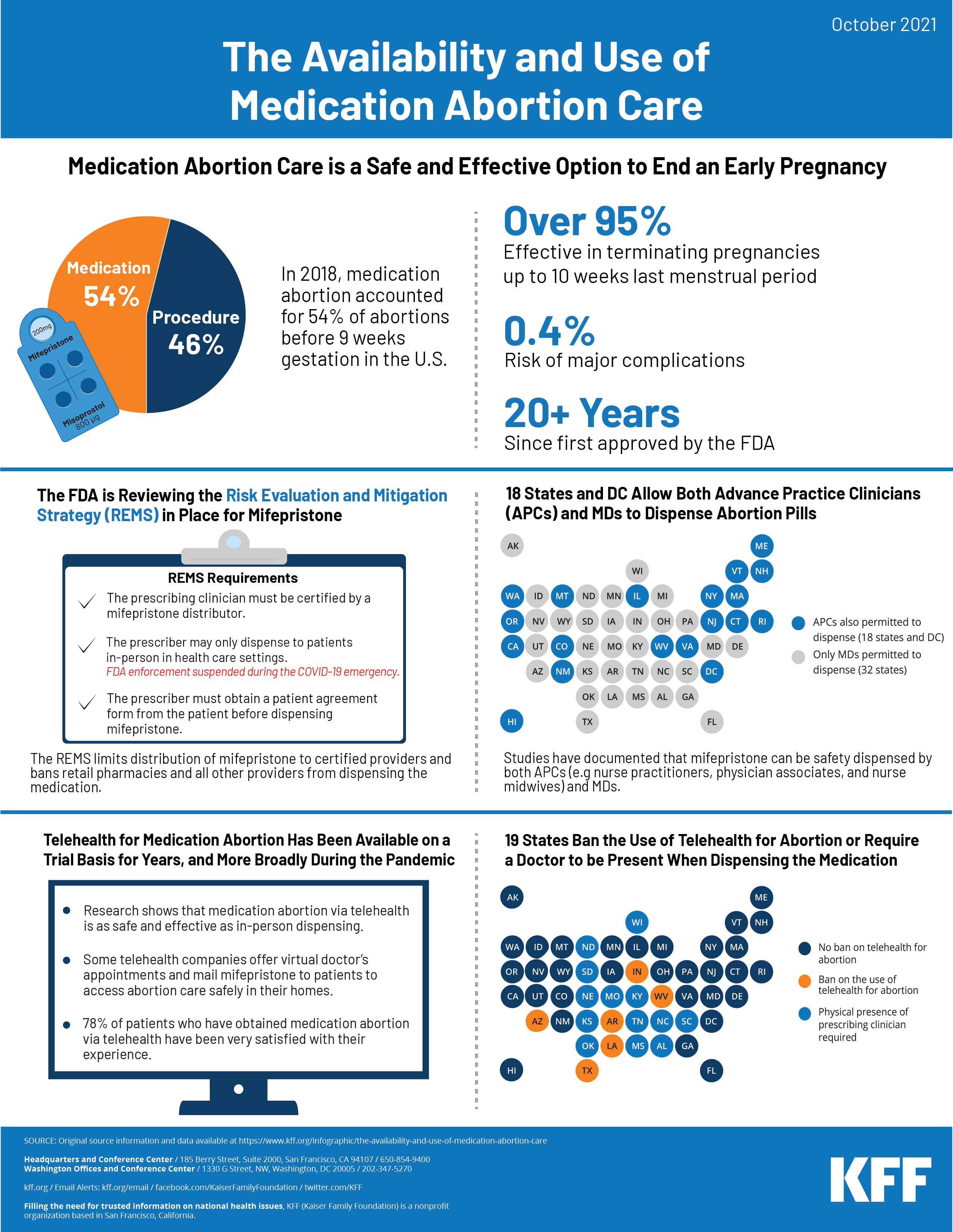
Medication abortion, also known as medical abortion or abortion with pills, is an FDA approved pregnancy termination protocol that involves taking two different drugs, mifepristone and misoprostol, for use up to the first 70 days (10 weeks) of pregnancy. Studies show that medication abortion care is safe and effective. This infographic highlights data and policies regarding the availability and effectiveness of medication abortion in the United States. Medication abortion accounts for more than half (54%) of all abortions before nine weeks gestation in the United States.
On April 12, 2021 , the FDA’s Center for Drug Evaluation and Research notified the American College of Obstetricians and Gynecologists (ACOG) that they are suspending enforcement of the Risk Evaluation and Mitigation Strategy (REMS) requirement for mifepristone that requires prescribers to dispense to patients in-person during the COVID-19 emergency. This temporarily allows providers in the 32 states and DC that do not have laws that otherwise ban this practice to dispense mifepristone using the telehealth protocol for medication abortion. The FDA has also undertaken a full review of the REMS for mifepristone and the results of that review are expected later in 2021.
.
View Source Slides
