Explaining the Prescription Drug Provisions in the Inflation Reduction Act
The Inflation Reduction Act of 2022, signed into law by President Biden on August 16, 2022, includes several provisions to lower prescription drug costs for people with Medicare and reduce drug spending by the federal government. This legislation has taken shape amidst strong bipartisan, public support for the government to address high and rising drug prices. CBO estimates that the drug pricing provisions in the law will reduce the federal deficit by $237 billion over 10 years (2022-2031).
The prescription drug provisions included in the Inflation Reduction Act will:
- Require the federal government to negotiate prices for some drugs covered under Medicare Part B and Part D with the highest total spending, beginning in 2026
- Require drug companies to pay rebates to Medicare if prices rise faster than inflation for drugs used by Medicare beneficiaries, beginning in 2023
- Cap out-of-pocket spending for Medicare Part D enrollees and make other Part D benefit design changes, beginning in 2024
- Limit monthly cost sharing for insulin to $35 for people with Medicare, beginning in 2023
- Eliminate cost sharing for adult vaccines covered under Medicare Part D and improve access to adult vaccines in Medicaid and CHIP, beginning in 2023
- Expand eligibility for full benefits under the Medicare Part D Low-Income Subsidy Program, beginning in 2024
- Further delay implementation of the Trump Administration’s drug rebate rule, beginning in 2027
This brief summarizes these provisions and discusses the expected effects on people, program spending, and drug prices and innovation.
Require the Federal Government to Negotiate Prices for Some Drugs Covered Under Medicare
Under the Medicare Part D program, which covers retail prescription drugs, Medicare contracts with private plan sponsors to provide a prescription drug benefit. The law that established the Part D benefit included a provision known as the “noninterference” clause, which stipulates that the HHS Secretary “may not interfere with the negotiations between drug manufacturers and pharmacies and PDP [prescription drug plan] sponsors, and may not require a particular formulary or institute a price structure for the reimbursement of covered part D drugs.” In addition, the Secretary of HHS does not currently negotiate prices for drugs covered under Medicare Part B (administered by physicians). Instead, Medicare reimburses providers based on a formula set at 106% of the Average Sales Price (ASP), which is the average price to all non-federal purchasers in the U.S, inclusive of rebates (other than rebates paid under the Medicaid program).
The Part D non-interference clause has been a longstanding target for some policymakers because it has limited the ability of the federal government to leverage lower prices, particularly for high-priced drugs without competitors. Medicare Part D and Part B drug spending is highly concentrated among a relatively small share of covered drugs, mainly those without generic or biosimilar competitors. A recent KFF Tracking Poll finds large majorities support allowing the federal government to negotiate drug prices and this support holds steady even after the public is provided with the arguments that were made for and against this proposal.
Provision Description
The Inflation Reduction Act amends the non-interference clause by adding an exception that requires the Secretary of HHS to negotiate prices with drug companies for a small number of single-source brand-name drugs or biologics without generic or biosimilar competitors that are covered under Medicare Part D (starting in 2026) and Part B (starting in 2028). Under the new Drug Price Negotiation Program, the number of drugs selected for price negotiation is 10 Part D drugs for 2026, another 15 Part D drugs for 2027, another 15 Part D and Part B drugs for 2028, and another 20 Part D and Part B drugs for 2029 and later years. These drugs will be selected from the 50 drugs with the highest total Medicare Part D spending and the 50 drugs with the highest total Medicare Part B spending. The number of drugs with negotiated prices available will accumulate over time.
Certain categories of drugs are excluded from the negotiation process, including:
- Drugs that have a generic or biosimilar available
- Drugs that are less than 9 years (for small-molecule drugs) or 13 years (for biological products) from their FDA-approval or licensure date
- “Small biotech drugs” (until 2029), defined as those which account for 1% or less of Part D or Part B spending and account for 80% or more of spending under each part on that manufacturer’s drugs
- Drugs with Medicare spending of less than $200 million in 2021 (increased by the CPI-U for subsequent years)
- Drugs with an orphan designation as their only FDA-approved indication
- All plasma-derived products
The legislation also delays selection of biologic drugs for negotiation by up to two years if a biosimilar product is likely to enter the market in that time.
The law establishes an upper limit for the negotiated price (the “maximum fair price”) for a given drug. The limit is the lower of the drug’s enrollment-weighted negotiated price (net of all price concessions) for a Part D drug, the average sales price for a Part B drug, or a percentage of a drug’s average non-federal average manufacturer price: 75% for small-molecule drugs and vaccines more than 9 years but less than 12 years beyond approval; 65% for drugs between 12 and 16 years beyond approval or licensure; and 40% for drugs more than 16 years beyond approval or licensure.
When negotiating the “maximum fair price” for a drug, the HHS Secretary is required to consider the following criteria:
- The manufacturer’s research and development costs, including the extent to which the manufacturer has recouped these costs
- The current unit costs of production and distribution
- Federal financial support for novel therapeutic discovery and development related to the drug
- Data on pending and approved patent applications, exclusivities, and certain other applications and approvals
- Market data and revenue and sales volume data in the US
- Evidence about alternative treatments, including:
- The extent to which the drug represents a therapeutic advance as compared to existing therapeutic alternatives and the costs of these alternatives
- Prescribing information for the drug and its therapeutic alternatives
- Comparative effectiveness of the drug and its therapeutic alternatives, taking into accounts their effects on specific populations, such as individuals with disabilities, the elderly, the terminally ill, children, and other patient populations
- The extent to which the drug and its therapeutic alternatives address unmet needs for a condition that is not adequately addressed by available therapy.
The law explicitly directs that the HHS Secretary “shall not use evidence from comparative clinical effectiveness research in a manner that treats extending the life of an elderly, disabled, or terminally ill individual as of lower value than extending the life of an individual who is younger, non-disabled, or not terminally ill.”
Part D drugs with negotiated “maximum fair prices” are required to be covered by all Part D plans. Medicare’s payment to providers for Part B drugs with negotiated prices will be 106% of the maximum fair price (rather than the current payment of 106% of the average sales price). (A separate section of the law increases Medicare payments to providers for the administration of biosimilar biologic products to 108% of the average sales price from October 1, 2022 through December 31, 2027.)
An excise tax will be levied on drug companies that do not comply with the negotiation process. The excise tax starts at 65% of a product’s sales in the U.S. and increases by 10% every quarter to a maximum of 95%. As an alternative to paying the tax, manufacturers can choose to withdraw all of their drugs from coverage under Medicare and Medicaid. In addition, manufacturers that refuse to offer an agreed-upon negotiated price for a selected drug to “a maximum fair price eligible individual” (i.e., Medicare beneficiaries enrolled in Part B and/or Part D) or to a provider of services to maximum fair price eligible individuals (such as a physician or hospital) will pay a civil monetary penalty equal to 10 times the difference between the price charged and the maximum fair price.
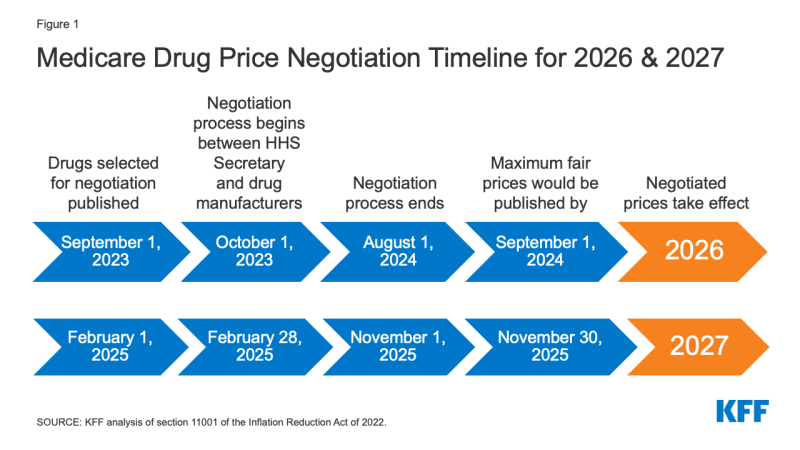
The timeline for the negotiation process spans roughly two years, although the timeline is modified for 2026, the first year that negotiated prices will be available under this new program (Figure 1). For the 10 Part D drugs with negotiated prices taking effect on January 1, 2026, the list of 10 Part D drugs selected for negotiation will be published on September 1, 2023, based on spending data for the 12-month period from June 1, 2022 to May 31, 2023. The period of negotiation between the Secretary and manufacturers of these drugs will occur between October 1, 2023 and August 1, 2024, and the negotiated “maximum fair prices” will be published no later than September 1, 2024. For 2027, which is an example of timing for a typical year in terms of the timeline for establishing negotiated prices, the list of 15 Part D drugs selected for negotiation will be published on February 1, 2025. The period of negotiation between the Secretary and manufacturers of the selected drugs will occur between February 28, 2025 and November 1, 2025 and the negotiated “maximum fair prices” will be published no later than November 30, 2025. For Part B drugs, the initial period of drug price negotiation between the Secretary and manufacturers of selected drugs will take place between February 28, 2026 and November 1, 2026, with negotiated prices first available in 2028.
The legislation appropriates funding of $3 billion in fiscal year 2022 for implementing the drug price negotiation provisions over the 2023-2031 period.
Effective Date
Negotiated prices for the first set of selected drugs covered under Part D will be available in 2026. For drugs covered under Part B, the first year negotiated prices will be available is 2028.
People affected
The provision to allow the Secretary to negotiate drug prices will put downward pressure on both Part D premiums and out-of-pocket drug costs, although the number of Medicare beneficiaries who will see lower out-of-pocket drug costs in any given year under the drug price negotiation program and the magnitude of savings will depend on how many and which drugs are subject to the negotiation process and the price reductions achieved through the negotiations process relative to what prices would otherwise be.
budgetary impact
CBO estimates $98.5 billion in Medicare savings over 10 years (2022-2031) from the drug negotiation provisions in the Inflation Reduction Act.
Effects on the Development of New Drugs
CBO estimates that the drug pricing provisions in the Inflation Reduction Act, including but not limited to the new Medicare drug price negotiation program, will have a very modest impact on the number of new drugs coming to market in the U.S. over the next 30 years: 13 fewer out of 1,300, or a reduction of 1% (about 1 fewer drug over the 2023-2032 period, about 5 fewer drugs in the subsequent decade, and about 7 fewer drugs in the decade after that).
Require Drug Manufacturers to Pay Rebates for Price Increases Above Inflation for Drugs Used by People with Medicare
To date, Medicare has had no authority to limit annual price increases for drugs covered under Part B or Part D. In contrast, Medicaid has a rebate system that requires drug manufacturers to provide refunds if prices grow faster than inflation. Year-to-year drug price increases exceeding inflation are not uncommon and affect people with both Medicare and private insurance. Our analysis shows that half of all drugs covered by Medicare had list price increases that exceeded the rate of inflation between 2019 and 2020. A separate analysis by the HHS Office of Inspector General showed average sales price (ASP) increases exceeding inflation for 50 of 64 studied Part B drugs in 2015.
provision description
The Inflation Reduction Act requires drug manufacturers to pay a rebate to the federal government if prices for single-source drugs and biologicals covered under Medicare Part B and nearly all covered drugs under Part D increase faster than the rate of inflation (CPI-U). Price changes will be measured based on the average sales price for Part B drugs and the average manufacturer price for Part D drugs. If price increases are higher than inflation, manufacturers will be required to pay the difference in the form of a rebate to Medicare. The rebate amount is equal to the total number of units sold in Medicare multiplied by the amount, if any, by which a drug’s price in a given year exceeds the inflation-adjusted price. For Part B drugs with price increases greater than inflation, beneficiary coinsurance will be based on 20% of the drug’s lower inflation-adjusted price. The base year for measuring cumulative price changes relative to inflation is 2021.
Rebate dollars would be deposited in the Medicare Supplementary Medical Insurance (SMI) trust fund. Manufacturers that do not pay the required rebate amount will face a penalty equal to at least 125% of the original rebate amount.
The legislation appropriates 10-year (2022-2031) funding of $160 million to the Centers for Medicare & Medicaid Services (CMS) for implementing the inflation rebate provisions ($80 million for Part B and $80 million for Part D).
Effective Date
The Part D inflation rebate provision takes effect in 2022, the starting point for measuring drug price increases, with rebate payments required beginning in 2023. The Part B inflation rebate provision takes effect in 2023.
People affected
These provisions are expected to limit out-of-pocket drug spending growth for people with Medicare and put downward pressure on premiums by discouraging drug companies from increasing prices faster than inflation. The number of Medicare beneficiaries who will see lower out-of-pocket drug costs in any given year resulting from these provisions will depend on how many and which drugs have lower price increases and the magnitude of price reductions relative to what prices would otherwise be.
budgetary impact
CBO estimates a net federal deficit reduction of $63.2 billion over 10 years (2022-2031) from the drug inflation rebate provisions in the Inflation Reduction Act. This includes net savings of $56.3 billion ($71.8 billion in savings to Medicare and $0.3 billion in savings for other federal programs, such as DoD, FEHB, and subsides for ACA Marketplace coverage, offset by $15.7 billion in additional Medicaid spending) and higher federal revenues of $6.9 billion.
Effects on Launch Pricing
Drug manufacturers may respond to the inflation rebates by increasing launch prices for drugs that come to market in the future. CBO projects that higher launch prices would primarily affect Medicaid spending. This is because, although the basic Medicaid drug rebate would be larger (since it is calculated as a percentage of the average manufacturer price), the higher Medicaid drug rebates would not offset higher launch prices. According to CBO, Medicare Part D plan sponsors and private insurers would be less affected than Medicaid by higher launch prices because they would still be able to negotiate rebates with drug companies and potentially refuse to cover drugs with very high launch prices. However, they may have less leverage in some instances, such as when there are no therapeutic alternatives available or when drugs are covered under a Part D “protected class”. In addition, if launch prices rise for Part B drugs, the HHS Secretary would have no authority to negotiate lower prices unless and until the new drug meets the criteria for selection for drug price negotiation under the negotiation process described above.
Cap Out-of-Pocket Spending for Medicare Part D Enrollees and Other Part D Benefit Design Changes
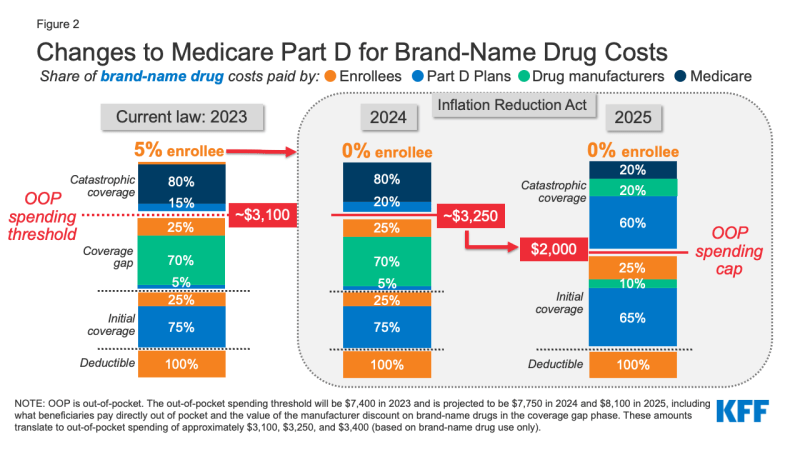
Medicare Part D currently provides catastrophic coverage for high out-of-pocket drug costs, but there is no limit on the total amount that beneficiaries pay out of pocket each year. Under the current benefit design, Part D enrollees qualify for catastrophic coverage when the amount that they pay out of pocket plus the value of the manufacturer discount on the price of brand-name drugs in the coverage gap phase exceeds a certain threshold amount. Enrollees with drug costs high enough to exceed the catastrophic threshold are required to pay 5% of their total drug costs above the threshold until the end of the year unless they qualify for Part D Low-Income Subsidies (LIS). In 2022, the catastrophic threshold is set at $7,050, and beneficiaries pay about $3,000 out of pocket for brand-name drugs before reaching the catastrophic coverage phase.
Medicare pays 80% of total costs above the catastrophic threshold (known as “reinsurance”) and plans pay 15%. Medicare’s reinsurance payments to Part D plans now account for close to half of total Part D spending (47%), up from 14% in 2006 (increasing from $6 billion in 2006 to $52 billion in 2021).
Under the current structure of Part D, there are multiple phases, including a deductible, an initial coverage phase, a coverage gap phase, and the catastrophic phase. During the coverage gap benefit phase, enrollees pay 25% of drug costs for both brand-name and generic drugs; plan sponsors pay 5% for brands and 75% for generics; and drug manufacturers provide a 70% price discount on brands (there is no discount on generics). Under the current benefit design, beneficiaries can face different cost-sharing amounts for the same medication depending on which phase of the benefit they are in, and can face significant out-of-pocket costs for high-priced drugs because of coinsurance requirements and no hard out-of-pocket cap.
provision description
The Inflation Reduction Act amends the design of the Part D benefit. For 2024, the law eliminates the 5% beneficiary coinsurance requirement above the catastrophic coverage threshold, effectively capping out-of-pocket costs at approximately $3,250 that year. Beginning in 2025, the legislation adds a hard cap on out-of-pocket spending of $2,000, indexed in future years to the rate of increase in per capita Part D costs (Figure 2).
The law also modifies liability for Medicare Part D plans and drug manufacturers, starting in 2025, and reduces Medicare’s liability for spending above the out-of-pocket cap. Medicare’s share of total costs above the spending cap (“reinsurance”) will decrease from 80% to 20% for brand-name drugs and to 40% for generic drugs. Medicare Part D plans’ share of costs will increase from 15% to 60% for both brands and generics above the cap, and drug manufacturers will be required to provide a 20% price discount on brand-name drugs. The legislation also requires manufacturers to provide a 10% discount on brand-name drugs between the deductible and the annual out-of-pocket spending cap, replacing the 70% price discount in the coverage gap phase under the current benefit design.
The law also provides for an adjustment to the calculation of the base beneficiary premium for 2024 through 2029, limiting premium increases to no more than 6% from the prior year. For 2030, the bill includes a provision to lower the beneficiary share of the cost of standard drug coverage (currently set at 25.5%) to ensure that the premium does not increase by more than 6% from 2029. The legislation also allows Part D enrollees the option of spreading out their out-of-pocket costs over the year rather than face high out-of-pocket costs in any given month.
Effective Date
The Part D benefit redesign provisions take effect beginning in 2024, with the elimination of the 5% coinsurance for catastrophic coverage and the first year of the Part D premium adjustment. Other changes take effect in 2025, including the $2,000 cap on out-of-pocket drug spending, spreading out of costs, and changes to liability for total costs above the spending cap.
people affected
Medicare beneficiaries in Part D plans with relatively high out-of-pocket drug costs are likely to see substantial out-of-pocket cost savings from these changes. This includes Medicare beneficiaries with spending above the catastrophic threshold due to just one very high-priced specialty drug for medical conditions such as cancer, hepatitis C, or multiple sclerosis and beneficiaries who take a handful of relatively costly brand or specialty medications to manage their medical conditions.
Based on our analysis, 1.4 million Part D enrollees incurred annual out-of-pocket costs for their medications above $2,000 in 2020, averaging $3,355 per person. This estimate includes 1.3 million enrollees who had spending above the catastrophic coverage threshold (which equaled roughly $2,700 in out-of-pocket costs that year for brand-name drugs alone). These estimates are a conservative measure of how many beneficiaries will be helped by capping out-of-pocket drug spending under Medicare Part D starting in 2024 because they do not account for expected increases in annual out-of-pocket drug spending between 2020 and 2024/2025, the increase in the number of beneficiaries on Medicare, or higher utilization and spending associated with the increased affordability of prescription drugs due to this benefit improvement.
Based on their average out-of-pocket spending, these 1.4 million Part D enrollees would have saved $1,355, or 40% of their annual out-of-pocket costs, on average, if a $2,000 cap had been in place in 2020. Part D enrollees with higher-than-average out-of-pocket costs will save substantial amounts with a $2,000 out-of-pocket spending cap. For example, the top 10% of beneficiaries (145,000 enrollees) with average out-of-pocket costs for their medications above $2,000 in 2020 – who spent at least $5,567 – would have saved $3,567 (64%) in out-of-pocket costs with a $2,000 cap.
Capping out-of-pocket drug spending under Medicare Part D will be especially helpful for beneficiaries who take high-priced drugs for conditions such as cancer or multiple sclerosis. For example, in 2020, among Part D enrollees without low-income subsidies, average annual out-of-pocket spending for the cancer drug Revlimid was $6,200 (used by 33,000 beneficiaries); $5,700 for the cancer drug Imbruvica (used by 21,000 beneficiaries); and $4,100 for the MS drug Avonex (used by 2,000 beneficiaries).
With the new hard cap on out-of-pocket spending, it is possible that enrollees could face higher Part D premiums resulting from higher plan liability for drug costs above the spending cap, though these premium increases could be mitigated by the provisions to stabilize premiums between 2024 and 2030. Plans will likely face financial incentives to exercise greater control of costs below the new spending cap, such as through more utilization management or increased generic drug utilization, which could help to limit potential premium increases.
budgetary impact
CBO estimates these provisions will increase federal spending by $30 billion over 10 years (2022-2031), which consists of $29.9 billion in higher spending associated with Part D benefit redesign and $0.1 billion in higher spending associated with the provision to spread out out-of-pocket costs.
Limit Cost Sharing for Insulin for People with Medicare
For Medicare beneficiaries with diabetes who use insulin, coverage is provided under Medicare Part D, the outpatient prescription drug benefit, and may also be covered under Part B when used with an external insulin pump. Because Part D plans vary in terms of the insulin products they cover and costs per prescription, what enrollees pay for insulin products also varies. Beneficiary coinsurance under Medicare Part B is 20% of the Medicare-approved amount.
Currently, Medicare beneficiaries can choose to enroll in a Part D plan participating in an Innovation Center model in which enhanced drug plans cover insulin products at a monthly copayment of $35 in the deductible, initial coverage, and coverage gap phases of the Part D benefit. Participating plans do not have to cover all insulin products at the $35 monthly copayment amount, just one of each dosage form (vial, pen) and insulin type (rapid-acting, short-acting, intermediate-acting, and long-acting). In 2022, a total of 2,159 Part D plans are participating in this model, or roughly one third of all Part D plans. Nearly half (45%) of non-LIS enrollees are in PDPs participating in the insulin model in 2022, based on August 2021 enrollment. The model was launched in response to rising prices for insulin, which have attracted increasing scrutiny from policymakers, leading to congressional investigations and overall concerns about affordability and access for people with diabetes who need insulin to control blood glucose levels.
provision description
The Inflation Reduction Act limits monthly cost sharing for insulin products to no more than $35 for Medicare beneficiaries, including insulin covered under both Part D and Part B, and no deductible will apply. All Medicare Part D plans, both stand-alone drug plans and Medicare Advantage drug plans, will be required to charge no more than $35 for whichever insulin products they cover, although plans will not be required to cover all insulin products. For 2026 and beyond, the law limits monthly Part D copayments for insulin to the lesser of $35, 25% of the maximum fair price (in cases where the insulin product has been selected for negotiation), or 25% of the negotiated price in Part D plans.
Effective Date
The monthly cap on insulin cost sharing in Medicare takes effect January 1, 2023 for insulin covered under Part D and July 1, 2023 for insulin covered under Part B.
People affected
A $35 cap on monthly cost sharing for insulin products is expected to lower out-of-pocket costs for insulin users in Medicare Part D without low-income subsidies. In 2020, 3.3 million Medicare Part D enrollees used insulin. Among Medicare Part D insulin users who do not receive low-income subsidies, average out-of-pocket costs per prescription across all insulin products was $54 in 2020 – over 50% more than the $35 monthly copay cap for insulin that will begin in 2023.
According to our analysis of 2019 Part D formularies, a large number of Part D plans placed insulin products on Tier 3, the preferred drug tier, which typically had a $47 copayment per prescription during the initial coverage phase. However, once enrollees reached the coverage gap phase, they faced a 25% coinsurance rate, which equates to $100 or more per prescription in out-of-pocket costs for many insulin therapies, unless they qualified for low-income subsidies. Paying a flat $35 copayment rather than 25% coinsurance or a higher copayment amount could reduce out-of-pocket costs for many insulin products.
budgetary impact
CBO estimates additional federal spending of $5.1 billion ($4.8 billion for Medicare Part D and $0.3 billion for Medicare Part B) over 10 years (2022-2031) associated with the insulin cost-sharing limits in the Inflation Reduction Act.
Eliminate Cost Sharing for Adult Vaccines Covered Under Part D and Improve Access to Adult Vaccines in Medicaid and CHIP
Medicare covers vaccines under both Part B and Part D. This separation of coverage for vaccines under Medicare is because there were statutory requirements for coverage of a small number of vaccines under Part B before the 2006 start of the Part D benefit. Vaccines for COVID-19, influenza, pneumococcal disease, and hepatitis B (for patients at high or intermediate risk), and vaccines needed to treat an injury or exposure to disease are covered under Part B. All other commercially available vaccines needed to prevent illness are covered under Medicare Part D.
For the influenza, pneumococcal pneumonia, hepatitis B, and COVID-19 vaccines covered under Medicare Part B, patients currently face no cost sharing for either the vaccine itself or its administration. For other Part B vaccines, such as those needed to treat an injury or exposure to a disease such as rabies or tetanus, Medicare covers 80% of the cost, and beneficiaries are responsible for the remaining 20%. Unlike most vaccines covered under Part B, vaccines covered under Part D can be subject to cost sharing, because Part D plans have flexibility to determine how much enrollees will be required to pay for any given on-formulary drug, including vaccines. (Part D enrollees who receive low-income subsidies (LIS) generally pay relatively low amounts for vaccines and other covered drugs.) Under Part D, cost sharing can take the form of flat dollar copayments or coinsurance (i.e., a percentage of list price).
With regard to Medicaid and CHIP, coverage of adult vaccines is optional and varies by state. According to a recent survey, half of states (25) did not cover all vaccines recommended by the Advisory Committee on Immunization Practices (ACIP) in 2018–2019, and 15 of 44 states responding to the survey imposed cost sharing requirements on adult vaccines.
provision description
The Inflation Reduction Act requires that adult vaccines covered under Medicare Part D that are recommended by the Advisory Committee on Immunization Practices (ACIP), such as for shingles, be covered at no cost. This makes coverage of vaccines under Medicare Part D consistent with coverage of vaccines under Medicare Part B, such as the flu and COVID-19 vaccines. The law also requires state Medicaid and CHIP programs to cover all approved adult vaccines recommended by ACIP and vaccine administration, without cost sharing.
Effective Date
These provisions take effect in 2023.
People affected
Eliminating cost-sharing for adult vaccines covered under Medicare Part D could help with vaccine uptake among older adults and will lower out-of-pocket costs for those who need Part D-covered vaccines. Our analysis shows that in 2020, 4.1 million Medicare beneficiaries received a Part D-covered vaccine, including 3.6 million who received the vaccine to prevent shingles, and aggregate out-of-pocket spending on Part D vaccines was $0.3 billion. In 2018, Part D enrollees without low-income subsidies paid an average of $57 out of pocket for each dose of the shingles shot, which is generally free to most other people with private coverage.
Requiring state Medicaid and CHIP programs to cover all adult vaccines recommended by ACIP without cost sharing is expected to increase access to some adult vaccines under Medicaid. Using a recent survey’s state level data and 2019 adult Medicaid enrollment data, a separate KFF analysis estimates about 4 million adults could gain coverage of at least one or more vaccines.
budgetary impact
CBO estimates that these provisions will increase federal spending by $7 billion over 10 years (2022-2031), including $4.4 billion for Medicare and $2.5 billion for Medicaid and CHIP.
Expand Eligibility for Part D Low-Income Subsidies
provision description
The Part D Low-Income Subsidy (LIS) Program helps beneficiaries with their Part D premiums, deductibles, and cost sharing, providing varying levels of assistance to beneficiaries at different income and asset levels up to 150% of poverty. Based on data from CMS, in 2020, 13.1 million Medicare beneficiaries received either full or partial LIS benefits, representing 28% of all Part D enrollees that year.
Medicare beneficiaries who are also enrolled in Medicaid, who generally have incomes up to 135% of poverty, automatically receive full LIS benefits. Individuals who do not automatically qualify for LIS can enroll if they meet certain income and asset requirements set by the federal government and can receive full or partial LIS benefits depending on their income and assets. Beneficiaries qualify for full LIS benefits if they have income up to 135% of poverty and resources up to $9,900 individual, $15,600 couple in 2022 (including a $1,500 per person allowance for funeral/burial expenses). Beneficiaries qualify for partial LIS benefits if they have income between 135-150% of poverty and resources up to $15,510 individual, $30,950 couple in 2022.
Beneficiaries who receive full LIS benefits pay no Part D premium or deductible and only modest copayments for prescription drugs until they reach the catastrophic threshold, at which point they face no additional cost sharing. Some beneficiaries who receive partial LIS benefits pay no monthly premium while others pay a partial monthly Part D premium (with subsidies of 75%, 50%, or 25% of the monthly premium, depending on their income); all partial LIS recipients also pay an $89 annual deductible (in 2022), 15% coinsurance up to the out-of-pocket threshold, and modest copayments for drugs above the catastrophic threshold.
The Inflation Reduction Act makes individuals with incomes up to 150% of poverty and resources at or below the limits for partial LIS benefits eligible for full benefits under the Part D Low-Income Subsidy Program. The law eliminates the partial LIS benefit currently in place for individuals with incomes between 135% and 150% of poverty.
Effective Date
Expansion of eligibility for full Part D LIS benefits takes effect in 2024.
People affected
Providing full Medicare Part D LIS benefits to Part D enrollees with incomes up to 150% of poverty could help an estimated 0.4 million beneficiaries, based on the number of beneficiaries receiving partial LIS benefits in 2020. Annual out-of-pocket drug costs for these beneficiaries could fall by close to $300, on average, based on the difference between average out-of-pocket drug costs for LIS enrollees receiving full benefits versus partial benefits in 2020 – plus additional savings associated with more generous premium subsidies.
These averages understate the potential cost savings for the smaller share of low-income enrollees with extraordinarily high drug costs, such as partial LIS beneficiaries who take high-cost specialty drugs. This is because for high-cost drugs, with total prices in the thousands of dollars, 15% coinsurance can translate into substantial out-of-pocket costs. For example, partial LIS enrollees taking Humira or Enbrel for rheumatoid arthritis would pay around $1,900 for a year’s worth of these medications in 2022, while full LIS enrollees would pay less than $20 annually. Thus, savings for partial LIS enrollees would be roughly $1,900 on cost sharing for one of these medications alone. Annual savings would be similar for other high-cost specialty drugs, with the majority of savings occurring below the catastrophic threshold where partial LIS enrollees currently pay 15% coinsurance but full LIS enrollees pay low flat copays for brand-name drugs of either $3.95 or $9.85, depending on their income and asset levels.
budgetary impact
CBO estimates that this provision will increase federal spending by $2.2 billion over 10 years (2022-2031).
Further Delay Implementation of the Trump Administration’s Drug Rebate Rule
provision description
The Inflation Reduction Act further delays implementation of the November 2020 final rule issued by the Trump Administration that would have eliminated rebates negotiated between drug manufacturers and pharmacy benefit managers (PBMs) or health plan sponsors in Medicare Part D by removing the safe harbor protection currently extended to these rebate arrangements under the federal anti-kickback statute. This rule was slated to take effect on January 1, 2022, but the Biden Administration delayed implementation to 2023, the Infrastructure Investment and Jobs Act signed into law on November 15, 2021 delayed implementation to 2026, and the Bipartisan Safer Communities Act signed into law on June 25, 2022 included a further delay to 2027.
Effective Date
This provision takes effect in 2027, delaying implementation of the rebate rule until 2032.
People affected
Since the rebate rule never took effect, delaying it is not expected to have a material impact on Medicare beneficiaries. Had the rule taken effect, it was expected to increase premiums for Medicare Part D enrollees, according to both CBO and the HHS Office of the Actuary (OACT). OACT estimated that a small group of beneficiaries who use drugs with significant manufacturer rebates could have seen a substantial decline in their overall out-of-pocket spending under the rule, assuming manufacturers passed on price discounts at the point of sale, but other beneficiaries would have faced out-of-pocket cost increases.
budgetary impact
Because the rebate rule was finalized (although not implemented), its cost has been incorporated in CBO’s baseline for federal spending. Therefore, delaying implementation of the rebate rule is expected to generate savings. CBO estimates savings of $122.2 billion from delaying implementation of the Trump Administration’s rebate rule between 2027 (when the Inflation Reduction Act delay takes effect) and 2032. In addition, CBO estimated savings of $50.8 billion between 2023 and 2026 for the three-year delay of this rule included in the Infrastructure Investment and Jobs Act and savings of $20.9 billion in 2026 and 2027 for the one-year delay included in the Bipartisan Safer Communities Act. This is because both CBO and Medicare’s actuaries estimated substantially higher Medicare spending over 10 years as a result of banning drug rebates under the Trump Administration’s rule – up to $170 billion higher, according to CBO, and up to $196 billion higher, according to the HHS Office of the Actuary (OACT).